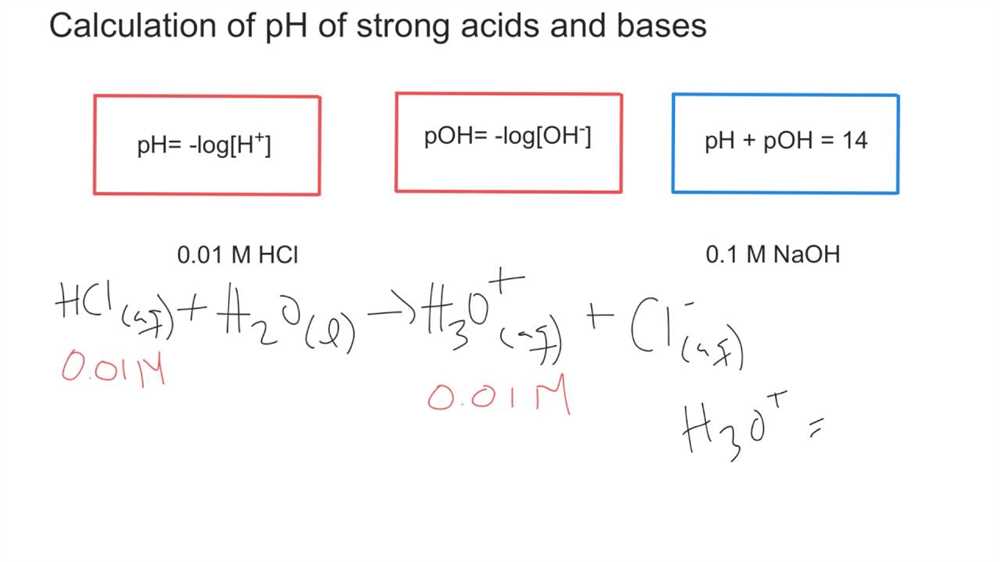
In chemistry, pH and pOH are two important measures used to describe the acidity or alkalinity (basicity) of a solution. The pH scale measures the concentration of hydrogen ions (H+) in a solution, while the pOH scale measures the concentration of hydroxide ions (OH-) in a solution. Both scales are logarithmic, meaning that each unit represents a tenfold difference in concentration.
To calculate the pH of a solution, the negative logarithm of the hydrogen ion concentration is taken. The formula is pH = -log[H+]. Conversely, to calculate the pOH of a solution, the negative logarithm of the hydroxide ion concentration is taken. The formula is pOH = -log[OH-]. These calculations allow chemists to quantify acidity and alkalinity and compare the relative strengths of different solutions.
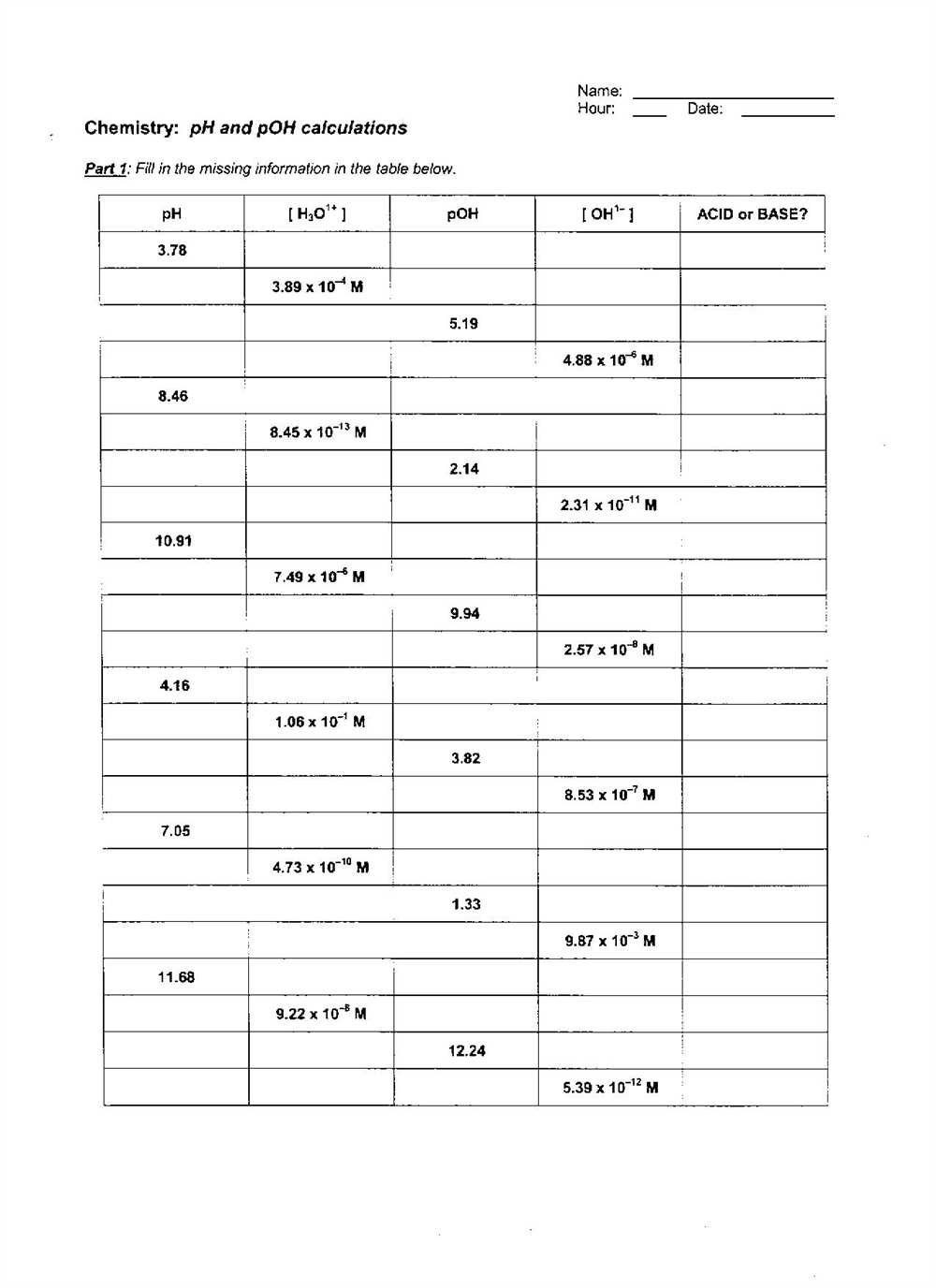
Calculating pH and pOH values can be done using a variety of methods, such as using pH paper or a pH meter. However, in a worksheet, calculations are typically done using the known concentrations of hydrogen ions and hydroxide ions. The answers to a pH and pOH worksheet typically involve plugging in the given values into the respective formulas and solving for the pH or pOH.
By practicing these calculations, students can improve their understanding of pH and pOH and strengthen their analytical skills. The answers to a pH and pOH worksheet can provide valuable feedback for students, allowing them to check their work and identify any areas where further study may be needed. Additionally, these calculations are often used in various fields of science, including environmental science, biology, and medicine, making them an important skill for future scientists to master.
Calculating pH and pOH Worksheet Answers
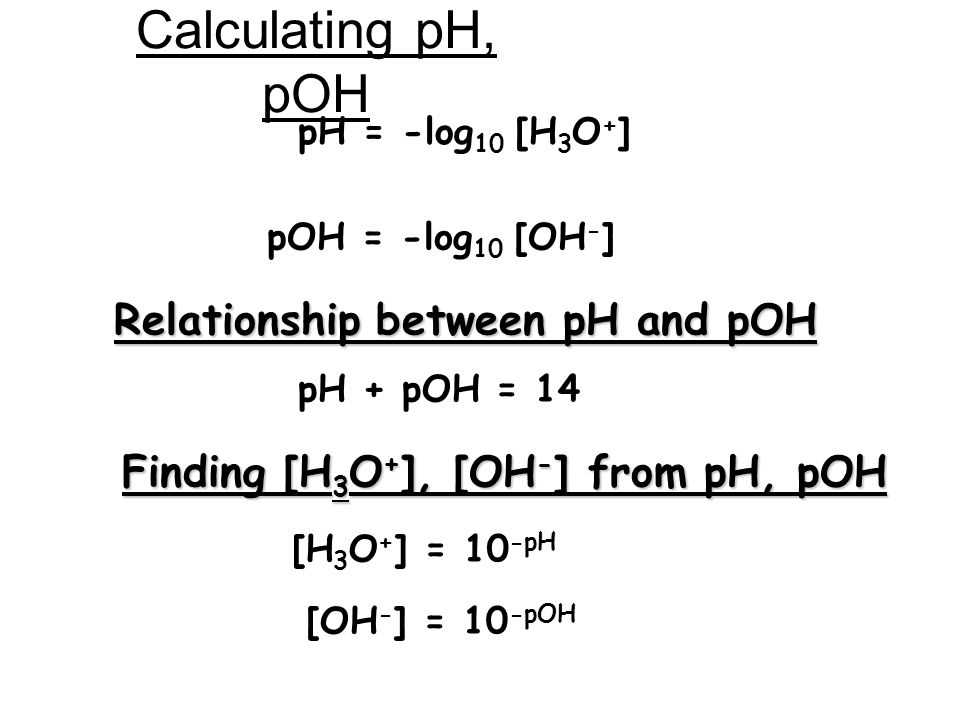
Calculating pH and pOH are fundamental skills in understanding the acidity or basicity of a solution. This worksheet provides answers to common questions related to these calculations.
1. How do I calculate the pH of a solution?
To calculate the pH of a solution, you need to take the negative logarithm (base 10) of the hydrogen ion concentration ([H+]). The formula is pH = -log[H+]. For example, if the [H+] is 1 × 10^-4 M, the pH would be 4.
2. How do I calculate the pOH of a solution?
The pOH is calculated in a similar way to the pH, but it represents the negative logarithm of the hydroxide ion concentration ([OH-]). The formula is pOH = -log[OH-]. For example, if the [OH-] is 1 × 10^-3 M, the pOH would be 3.
3. How can I convert between pH and pOH?
Since pH and pOH represent different ions (H+ and OH-), they are related to each other by the equation pH + pOH = 14. This means that if you know the pH, you can calculate the pOH by subtracting the pH from 14, or vice versa.
4. What is the relationship between pH and acidity?
The pH scale ranges from 0 to 14, with values below 7 indicating acidity, 7 representing neutrality, and values above 7 indicating basicity. The lower the pH value, the stronger the acid; conversely, the higher the pH value, the stronger the base.
5. Can I calculate the pH of a strong acid or base without using the concentration?
Yes, for strong acids or bases, the pH can be determined solely based on the chemical formula. Strong acids, such as hydrochloric acid (HCl), completely dissociate in water, resulting in a large concentration of H+ ions. Similarly, strong bases, like sodium hydroxide (NaOH), dissociate completely to give a high concentration of OH- ions. Therefore, knowing the formula of a strong acid or base allows you to determine the pH without explicitly measuring the concentration.
- Overall, calculating pH and pOH is crucial for understanding the acidity or basicity of a solution. pH represents the hydrogen ion concentration, while pOH represents the hydroxide ion concentration. These values can be converted between each other using the equation pH + pOH = 14. The pH scale ranges from 0 to 14, with lower values indicating acidity and higher values indicating basicity. For strong acids or bases, the pH can be determined solely based on the chemical formula.
pH and pOH: Understanding the Basics
In chemistry, pH and pOH are important concepts used to measure the acidity or alkalinity of a solution. Understanding the basics of pH and pOH is crucial for various applications in the field of chemistry.
pH: pH is a measure of the hydrogen ion concentration in a solution. It is a logarithmic scale that ranges from 0 to 14, with 7 being considered neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are alkaline or basic.
To calculate the pH of a solution, you can use the equation:
pH = -log[H+]
Where [H+] represents the concentration of hydrogen ions in moles per liter. This equation allows you to determine the acidity or alkalinity of a solution by measuring the concentration of hydrogen ions.
pOH: pOH is another measure of the acidity or alkalinity of a solution, but it focuses on the concentration of hydroxide ions instead of hydrogen ions. Like pH, pOH is also a logarithmic scale that ranges from 0 to 14. A solution with a pOH less than 7 is considered basic, while a pOH greater than 7 is considered acidic.
The equation to calculate pOH is:
pOH = -log[OH-]
Here, [OH-] represents the concentration of hydroxide ions in moles per liter. By calculating the pOH of a solution, you can determine its alkalinity or acidity based on the concentration of hydroxide ions.
It is important to note that pH and pOH are related to each other. The sum of pH and pOH will always be equal to 14, indicating the presence of neutral water.
Understanding pH and pOH is fundamental for conducting experiments, analyzing chemical reactions, and maintaining the correct conditions in various chemical processes.
The pH Scale: Exploring Acidic and Basic Solutions
In chemistry, the pH scale is a measure of the acidity or basicity of a solution. It is a logarithmic scale that ranges from 0 to 14. A pH of 7 is considered neutral, while pH values below 7 indicate acidity, and pH values above 7 indicate alkalinity. The pH scale is an important tool for scientists to quantify and compare the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in a solution.
The pH of a solution can be determined using various methods, such as pH paper, pH meters, or indicators. pH paper is impregnated with a chemical indicator that changes color based on the pH of the solution. pH meters are electronic devices that measure the voltage of a solution and convert it to a pH value. Indicators are chemical compounds that change color depending on the pH of the solution they are added to.
Acidic solutions have a pH below 7 and contain a higher concentration of hydrogen ions (H+). Examples of acidic substances include lemon juice, vinegar, and stomach acid. Basic solutions have a pH above 7 and contain a higher concentration of hydroxide ions (OH-). Examples of basic substances include soap, baking soda, and bleach. Neutral solutions have a pH of 7 and contain an equal concentration of hydrogen ions and hydroxide ions.
The pH scale is logarithmic, meaning that each whole number decrease on the scale represents a tenfold increase in acidity, while each whole number increase represents a tenfold increase in alkalinity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
Understanding the pH scale is important in many fields, including biology, environmental science, and medicine. It allows scientists to measure and control the acidity or alkalinity of solutions, which can have a significant impact on chemical reactions, biological processes, and the overall health of living organisms.
How to Calculate pH: Step-by-Step Guide
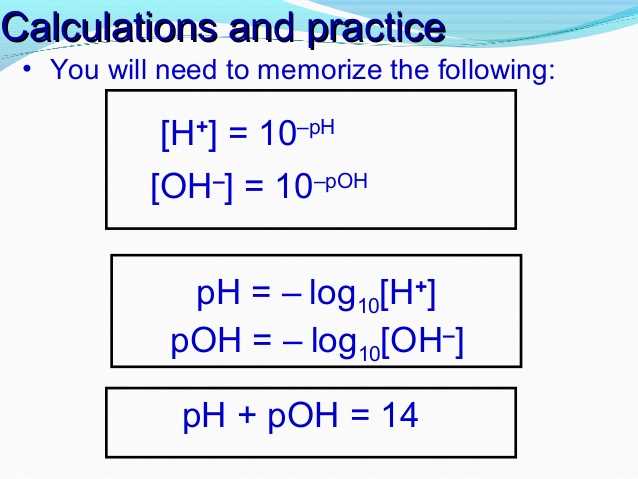
Calculating pH is an essential skill in chemistry, as it measures the acidity or alkalinity of a solution. pH stands for “potential of hydrogen” and is a logarithmic scale ranging from 0 to 14. A pH value below 7 indicates acidity, while a pH value above 7 indicates alkalinity. pH can be calculated using the concentration of hydrogen ions (H+) in the solution.
Here is a step-by-step guide on how to calculate pH:
- Determine the concentration of H+: You will need to know the molar concentration of hydrogen ions in the solution. This information is usually provided in the problem or can be measured experimentally.
- Convert concentration to scientific notation: If the concentration is not already given in scientific notation, convert it. For example, if the concentration is 0.001 M, convert it to 1 x 10^-3 M.
- Take the negative logarithm: Take the negative logarithm of the concentration using base 10. This can be done using a scientific calculator or by using logarithm tables. The formula is pH = -log[H+].
- Round to the appropriate number of decimal places: Round the pH value to the desired number of decimal places. Typically, pH is rounded to two decimal places.
By following these steps, you can calculate the pH of a solution. Remember that pH is a logarithmic scale, so even small changes in concentration can result in significant changes in pH.
Examples of pH Calculations: Practice Problems
pH calculations play a crucial role in understanding the level of acidity or alkalinity of substances. By calculating the pH of a solution, we can determine its concentration of hydrogen ions (H+) and classify it as either acidic, neutral, or basic. In order to master pH calculations, it is important to practice solving various problems. Here are some examples of practice problems:
Example 1:
Calculate the pH of a solution with a hydrogen ion concentration of 1.5 x 10^-3 M.
Solution:
To calculate the pH, we can use the equation: pH = -log[H+]. Substituting the given hydrogen ion concentration, we have pH = -log(1.5 x 10^-3) = 2.82.
Example 2:
Find the pH of a solution with a hydroxide ion concentration of 2.5 x 10^-9 M.
Solution:
In this case, we need to calculate the pOH first. The pOH can be determined using the equation: pOH = -log[OH-]. Substituting the given hydroxide ion concentration, we have pOH = -log(2.5 x 10^-9) = 8.60. Next, we can use the equation pH + pOH = 14 to find the pH. Since pH + pOH = 14, we have pH = 14 – pOH = 14 – 8.60 = 5.40.
Example 3:
Determine the hydrogen ion concentration of a solution with a pH of 3.75.
Solution:
To find the hydrogen ion concentration, we can use the equation: [H+] = 10^(-pH). Substituting the given pH value, we have [H+] = 10^(-3.75) = 1.78 x 10^(-4) M.
By practicing these types of pH calculations, you can enhance your understanding of the concept and improve your problem-solving skills. Remember to apply the appropriate equations and formulas to solve each problem effectively.
The Relationship Between pH and pOH
Understanding the relationship between pH and pOH is essential in the field of chemistry, particularly when working with acids and bases. pH and pOH are logarithmic scales used to measure the acidity or alkalinity of a solution. pH measures the concentration of hydrogen ions (H+) in a solution, while pOH measures the concentration of hydroxide ions (OH-).
The pH scale ranges from 0 to 14, with 7 being considered neutral. A pH value below 7 indicates acidity, while a pH value above 7 indicates alkalinity. The pOH scale is complementary to the pH scale, with pOH values below 7 indicating alkalinity and values above 7 indicating acidity.
The relationship between pH and pOH can be described by the equation: pH + pOH = 14. This means that if the pH of a solution is known, the pOH can be calculated by subtracting the pH value from 14, and vice versa. For example, if the pH of a solution is 3, the pOH can be calculated as 14 – 3 = 11.
Additionally, the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in a solution can be determined using the pH and pOH values. The concentration of H+ ions can be calculated using the equation: [H+] = 10^(-pH), while the concentration of OH- ions can be calculated using the equation: [OH-] = 10^(-pOH). These equations allow chemists to quantify the acidity or alkalinity of a solution and make calculations in various chemical reactions.
In conclusion, the relationship between pH and pOH is essential in determining the acidity or alkalinity of a solution. Understanding this relationship allows chemists to calculate pH and pOH values, as well as determine the concentration of hydrogen and hydroxide ions in a solution. This knowledge is crucial in many areas of chemistry, from analyzing acid-base reactions to understanding the properties of different substances.
How to Calculate pOH: Simplified Method
Calculating the pOH of a solution is an essential step in determining the acidity or basicity of a substance. The pOH scale is the negative logarithm of the concentration of hydroxide ions ([OH-]) in a solution. It is the basic counterpart to the pH scale, which measures the concentration of hydrogen ions ([H+]).
To calculate the pOH of a solution, you need to know the concentration of hydroxide ions. This information can usually be found in the chemical equation or given in the problem. If it is not given, you can determine the hydroxide ion concentration by using the concept of molarity. Molarity is defined as the moles of solute divided by the volume of solution in liters.
To simplify the process, you can use the power of 10 and the concept of pH. The equation for pH is pH = -log[H+]. Similarly, the equation for pOH is pOH = -log[OH-]. Since pH + pOH = 14 (for water at 25°C), you can calculate the pOH of a solution by subtracting the pH from 14.
For example, if the pH of a solution is 3, you can calculate the pOH as follows:
- pH = 3
- pOH = 14 – 3 = 11
Alternatively, if the hydroxide ion concentration is given in mol/L, you can use the direct equation pOH = -log[OH-]. In this case, you don’t need to calculate the pH first.
Remember, pOH is a logarithmic scale, so a lower pOH value indicates a higher concentration of hydroxide ions and a more basic solution. Conversely, a higher pOH value indicates a lower concentration of hydroxide ions and a more acidic solution.