
Equilibrium is a key concept in chemistry that refers to a state of balance between opposing forces or reactions. It is an important concept to understand as it plays a significant role in various chemical reactions and processes. In the laboratory, exploring equilibrium involves conducting experiments and analyzing the results to determine the conditions under which equilibrium is achieved.
One common laboratory experiment to explore equilibrium is the reaction between reactants A and B to form products C and D. By varying the concentrations of the reactants and observing the reaction over time, scientists can study the factors that influence the equilibrium position and the rate at which it is achieved.
Through this experimentation, scientists can obtain valuable data that can be used to calculate the equilibrium constant and determine how temperature, pressure, and concentration affect the equilibrium position. These results can then be used to predict and control chemical reactions in various applications, such as in industrial processes or in the development of new medications.
Understanding equilibrium and the factors that influence it is essential for chemists and researchers in order to optimize reaction conditions and maximize the yield of desired products. By exploring equilibrium in the laboratory and analyzing the resulting data, scientists are able to gain valuable insights into the behavior of chemical systems and make informed decisions in their research and application of chemistry.
Exploring Equilibrium Lab Answers: Understanding the Concept and Analyzing Results
In the field of chemistry, equilibrium is a fundamental concept that plays a crucial role in understanding chemical reactions and their behavior. Equilibrium refers to a state in which the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time. Exploring equilibrium in the laboratory setting allows students to gain a deeper understanding of this concept and its implications.
When conducting an equilibrium lab, students usually start with a set of initial conditions and carefully monitor the reaction to observe how it reaches a state of equilibrium. By measuring and recording the concentrations of reactants and products at various time intervals, they are able to analyze the data and draw meaningful conclusions about the reaction kinetics and the equilibrium position.
The lab answers obtained through exploring equilibrium involve interpreting data and making connections between the observed changes in concentrations and the principles of equilibrium. Students must demonstrate their understanding of factors that affect equilibrium, such as changes in temperature, concentration, and pressure. They analyze the data to identify trends, calculate reaction rates, and determine the equilibrium constants.
Furthermore, exploring equilibrium also allows students to develop their problem-solving and critical thinking skills. They must think analytically and logically to interpret the results accurately and draw valid conclusions. They may need to make predictions about the effects of changing certain parameters and propose possible explanations for any deviations from expected outcomes.
A successful exploration of equilibrium in the lab setting requires careful planning, accurate data collection, and meticulous analysis. By engaging in this process, students can deepen their understanding of equilibrium, enhance their scientific skills, and gain valuable experience in experimental design, data interpretation, and drawing conclusions based on evidence.
Equilibrium Lab Setup
In order to study chemical equilibrium in the laboratory, a carefully controlled setup is required. The experiment typically involves the reaction between two or more substances, which reach a state of equilibrium when the rates of the forward and reverse reactions are equal. The setup should allow for the measurement of reactant and product concentrations, as well as the ability to manipulate reaction conditions.
One common approach is to use a glass container, such as a test tube or a flask, where the reaction takes place. The container should be clean and transparent to easily observe any changes in the system. In addition, a stopper or cap may be used to prevent any gas exchange with the surroundings, if necessary.
To measure the reactant and product concentrations, various techniques can be employed. One method is to use spectrophotometry, which involves measuring the absorbance or transmittance of light passing through the solution. This can provide information about the concentration of specific compounds or ions in the solution. Another approach is titration, where a known concentration of a reagent is added to the solution to determine the concentration of a particular species.
Manipulating the reaction conditions is an important aspect of studying equilibrium. This can be achieved by varying parameters such as temperature, pressure, or concentration. A water bath or a heating mantle may be used to control the temperature, while a pressure regulator allows for adjusting the pressure if needed. The concentration of reactants can be modified by adding or removing appropriate amounts of substances.
Overall, a well-designed equilibrium lab setup enables scientists to conduct experiments and gather data that help to better understand the concept of equilibrium and its applications in chemical systems. By carefully controlling the experimental conditions and measuring the relevant quantities, researchers can analyze the behavior of the system at equilibrium and draw meaningful conclusions about the underlying chemical processes.
Experimental Procedure
The experimental procedure for exploring equilibrium in the lab involves several steps. Firstly, gather all the necessary materials and equipment, including test tubes, beakers, a spectrophotometer, and the chemicals needed for the specific reaction being studied.
Next, prepare the reaction mixture by carefully measuring and combining the appropriate amounts of reactants. This may involve mixing solutions or adding solid compounds to a solvent. It is crucial to accurately measure the quantities to ensure reliable results.
Once the reaction mixture is prepared, transfer it to a test tube or a suitable container. It is important to keep the temperature constant throughout the experiment, so place the test tube in a water bath if needed. This will help maintain the equilibrium state during the reaction.
To observe the changes in the reaction, take regular measurements using the spectrophotometer. This instrument measures the absorbance or the concentration of a specific component in the reaction mixture. Adjust the spectrophotometer accordingly and record the readings at regular intervals. This will provide valuable data about the reaction kinetics and how equilibrium is reached.
During the experiment, it is important to follow safety protocols, such as wearing gloves and goggles, and working in a well-ventilated area if toxic chemicals are being used. Additionally, repeat the experiment multiple times to ensure the results are consistent and reliable.
After the experiment, analyze the data and draw conclusions about the equilibrium state of the reaction. Compare the values obtained from the spectrophotometer readings and draw graphs to visualize the changes in concentration or absorbance over time. Discuss the results and possible sources of error in the experiment.
In conclusion, the experimental procedure for exploring equilibrium in the lab involves careful preparation of the reaction mixture, maintaining a constant temperature, taking regular measurements using a spectrophotometer, following safety protocols, and analyzing the data to draw conclusions about the equilibrium state. This process allows for a deeper understanding of the factors that influence equilibrium and how it can be reached.
Data Collection and Analysis
During the equilibrium lab, data collection and analysis play a crucial role in understanding the concepts and principles behind chemical equilibrium. By carefully collecting and analyzing data from the lab experiments, scientists can determine the conditions at which equilibrium is reached, and calculate the equilibrium constant.
Data Collection: In order to collect data, various measurements and observations are made throughout the lab experiment. These may include monitoring changes in temperature, pressure, concentration, and volume. Accurate and precise measurements are essential for obtaining reliable data. Using appropriate instruments and techniques, such as thermometers, pressure sensors, and volumetric equipment, ensures accurate data collection.
Data Analysis: Once the data is collected, it is analyzed to determine the equilibrium conditions and calculate the equilibrium constant. Data analysis involves examining the collected data for patterns and trends, identifying outliers or errors, and applying appropriate calculations and mathematical models. Statistical analysis may also be employed to determine the significance of the results and assess the reliability of the data.
There are several methods and techniques used to analyze equilibrium data. These include graphical analysis, such as plotting concentration versus time or pressure versus temperature curves, and mathematical analysis using equations derived from the principles of chemical equilibrium. By analyzing the data, scientists can draw conclusions about the behavior of the reacting species and the factors that influence the equilibrium position.
Overall, data collection and analysis are integral to the exploration of equilibrium in lab experiments. Through careful measurement and analysis, scientists can gain a deeper understanding of chemical equilibrium and its underlying principles. The data collected and analyzed during the lab experiments provide valuable insights into the factors that affect equilibrium and can contribute to the development of theories and models in the field of chemistry.
Factors Influencing Equilibrium
The equilibrium of a chemical reaction is influenced by various factors that can shift the balance between the reactants and products. These factors include changes in concentration, temperature, pressure, and the presence of catalysts.
1. Concentration:
Changing the concentration of reactants or products can have a significant impact on the equilibrium position. According to Le Chatelier’s principle, an increase in the concentration of a reactant will shift the equilibrium towards the formation of products, while an increase in the concentration of a product will favor the formation of reactants. Conversely, a decrease in the concentration of a reactant or product will have the opposite effect.
2. Temperature:
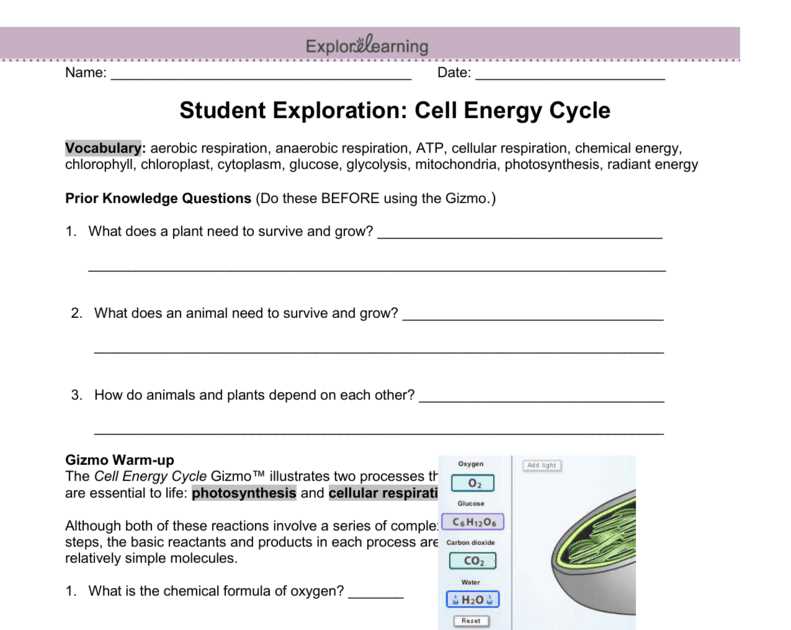
The temperature of a reaction also affects the equilibrium position. In an exothermic reaction, where heat is released, increasing the temperature will shift the equilibrium towards the reactants. Conversely, in an endothermic reaction, where heat is absorbed, increasing the temperature will favor the formation of products. Lowering the temperature will have the opposite effect for both types of reactions.
3. Pressure:

In reactions involving gases, changes in pressure can influence the equilibrium position. Increasing the pressure will shift the equilibrium towards the side with fewer moles of gas, while decreasing the pressure will favor the side with more moles of gas. This is based on the principle that molecules in a gas state occupy more volume compared to when they are in a liquid or solid state.
4. Catalysts:
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They do not directly affect the equilibrium position but can affect the time it takes for equilibrium to be reached. By lowering the activation energy of the reaction, catalysts can speed up both the forward and reverse reactions, allowing the system to reach equilibrium faster.
Overall, these factors play a crucial role in determining the equilibrium position of a chemical reaction. Understanding how they influence equilibrium can help scientists manipulate reaction conditions to achieve desired results in various industries, such as pharmaceuticals, food production, and environmental engineering.
Interpreting Lab Results
Interpreting lab results is a crucial part of any scientific experiment, as it allows researchers to draw meaningful conclusions from the data they have collected. In the context of exploring equilibrium, lab results can provide insights into the factors that affect the establishment and maintenance of equilibrium in a system.
When analyzing lab results related to equilibrium, it is important to look for key indicators such as the presence of equilibrium constants, reaction rates, and concentrations of reactants and products. These indicators can help identify whether equilibrium has been achieved, and if so, what factors influence the position of equilibrium.
Equilibrium constants play a significant role in interpreting lab results. The equilibrium constant, represented by the symbol K, is a ratio of the concentrations of products to reactants at equilibrium. It provides insight into the relative amounts of reactants and products in a system once equilibrium has been reached. A high value of K indicates that the system favors the formation of products, while a low value suggests a higher concentration of reactants. Therefore, when interpreting lab results, a thorough analysis of the equilibrium constant can reveal crucial information about the direction and extent of the reaction.
Reaction rates also play a pivotal role in interpreting lab results related to equilibrium. The rate at which a reaction progresses can provide insights into the kinetics of the system and the factors that influence the establishment of equilibrium. A faster reaction rate can indicate a system that is still far from equilibrium, while a slower reaction rate suggests that the system is closer to equilibrium. By analyzing the reaction rates at different stages of the experiment, researchers can gain a deeper understanding of how equilibrium is established and how it can be manipulated.
In conclusion, interpreting lab results requires a thorough analysis of various indicators such as equilibrium constants and reaction rates. These indicators provide valuable insights into the factors that influence the establishment and maintenance of equilibrium in a system. By examining these results, researchers can draw meaningful conclusions and further expand their understanding of equilibrium reactions.