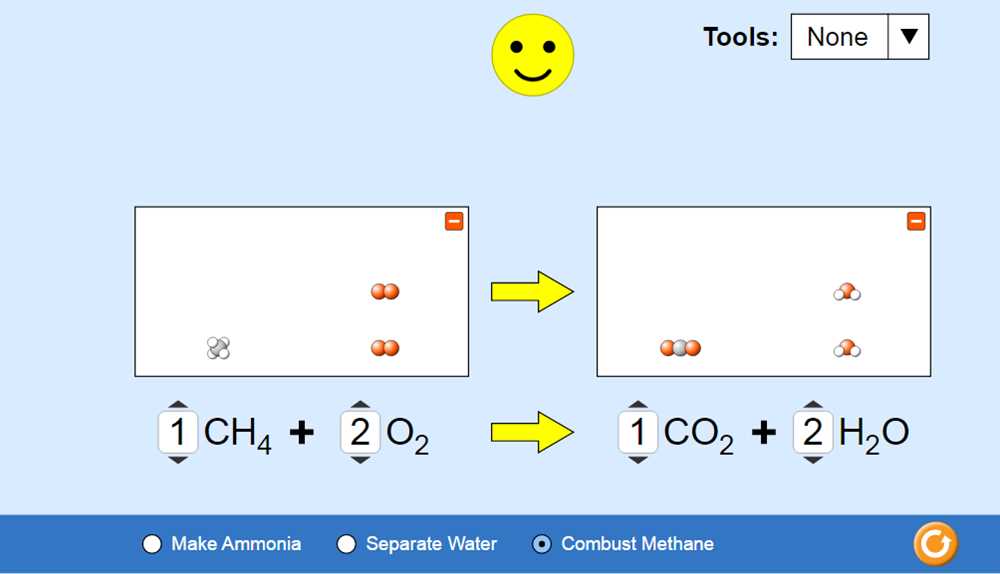
Stoichiometry is a fundamental concept in chemistry, playing a crucial role in understanding chemical reactions and predicting their outcomes. It is the study of the quantitative relationships between reactants and products in a chemical reaction. The PhET Lab on Stoichiometry is a virtual experiment that allows students to explore these principles and apply them to real-world scenarios.
In this lab, students are provided with a virtual laboratory setting where they can manipulate various reactants and observe the resulting products. By changing the amounts of reactants, they can observe how the stoichiometry of a reaction affects the quantities of products formed. Through interactive simulations, students can come to understand the concept of balanced chemical equations and stoichiometric calculations.
The lab also provides a series of guided questions and exercises that challenge students to apply their knowledge of stoichiometry to solve problems. By analyzing the reactant ratio in a chemical equation and using stoichiometric calculations, students can determine the theoretical yield, percent yield, and limiting reactant of a given reaction. These calculations not only reinforce the concept of stoichiometry but also provide practical insights into real-world applications of chemical reactions.
Overall, the Stoichiometry PhET Lab offers a hands-on and interactive learning experience that allows students to explore the principles of chemical reactions. By conducting virtual experiments and solving problems, students can develop a strong understanding of stoichiometry and its role in predicting the outcomes of chemical reactions. This lab serves as a valuable tool for educators and students alike in their pursuit of mastering the fundamental concepts of chemistry.
What is Stoichiometry?
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to determine the amount of reactants needed or the amount of products that can be formed in a chemical reaction, based on the balanced equation.
In order to understand stoichiometry, it is important to understand the concept of a balanced chemical equation. A balanced chemical equation represents a chemical reaction in which the number of atoms of each element is the same on both sides of the equation. This means that the mass is conserved during a chemical reaction, and the stoichiometric coefficients in the balanced equation represent the mole ratio between the reactants and products.
Stoichiometry calculations involve using the mole ratio from the balanced equation to determine the moles of reactants or products involved in a reaction. This information can then be used to calculate the mass, volume, or concentration of substances in the reaction. Stoichiometry is crucial in chemical calculations and is used in various fields, including pharmaceuticals, environmental science, and industrial processes.
In conclusion, stoichiometry is a vital tool in chemistry that allows us to quantitatively analyze chemical reactions. Through the use of balanced equations and mole ratios, stoichiometry enables us to determine the amounts of substances involved in a reaction, ultimately helping us understand and predict the outcomes of chemical reactions.
The Importance of Stoichiometry in Chemistry
Stoichiometry is a fundamental concept in chemistry that involves the quantitative relationships between reactants and products in a chemical reaction. It plays a crucial role in understanding and predicting the outcomes of chemical reactions and is essential for various applications in the field of chemistry.
Calculating Reactant and Product Quantities: Stoichiometry allows chemists to determine the exact quantities of reactants and products involved in a chemical reaction. By balancing the chemical equation and using stoichiometric calculations, scientists can calculate the amount of reactants needed for a desired yield or determine the amount of products that will be formed. This information is valuable for industries that rely on efficient production processes and for researchers aiming to optimize reactions.
Understanding Limiting Reactants: Stoichiometry helps chemists identify the limiting reactant in a chemical reaction, which is the reactant that gets consumed completely and limits the amount of product that can be formed. By determining the stoichiometric ratios, scientists can calculate the theoretical yield of a reaction, which represents the maximum amount of product that can be obtained. This knowledge allows for the optimization of reaction conditions and the identification of any potential inefficiencies.
Quantitative Analysis: Stoichiometry is also essential in quantitative analysis, where the amounts of substances in a sample are determined. By using stoichiometry, chemists can relate the amount of a reactant consumed or product formed to the amount of substance being analyzed. This information is crucial for various analytical techniques, such as titrations, gravimetric analysis, and instrumental methods, which rely on accurate measurements of quantities involved in a reaction.
Cross-Disciplinary Applications: Stoichiometry is not only important in the field of chemistry but also finds applications in other scientific disciplines. It is particularly relevant in environmental studies, where stoichiometric calculations help in understanding the composition and transformation of pollutants. Additionally, stoichiometry is utilized in biological systems to study metabolic pathways, enzyme kinetics, and nutrient cycling, providing insights into the functioning of living organisms.
Overall, stoichiometry plays a crucial role in chemistry by allowing for the calculation and prediction of reactant and product quantities, identifying limiting reactants, enabling quantitative analysis, and finding cross-disciplinary applications. Its importance lies in providing a quantitative understanding of chemical reactions and guiding the optimization of processes in various fields of study.
Understanding the PhET Lab
PhET Interactive Simulations is a platform that provides a wide range of interactive science simulations for students and educators. One of the popular simulations available on the platform is the Stoichiometry PhET Lab. This lab allows students to explore the concept of stoichiometry, which is the quantitative relationship between reactants and products in a chemical reaction.
Using the Stoichiometry PhET Lab, students can manipulate different variables such as the amount of reactants and the type of reaction to observe how these changes affect the quantities of products formed. The simulation provides a visual representation of the reaction, allowing students to easily understand and analyze the stoichiometry involved.
Through the lab, students can gain a deeper understanding of stoichiometry by experimenting with different scenarios and observing the changes in reactants and products. This hands-on approach helps students to grasp the concept more effectively and reinforces their understanding of stoichiometry in a practical way.
The Stoichiometry PhET Lab also includes pre-lab and post-lab questions to test students’ knowledge and comprehension of the topic. These questions require students to apply their understanding of stoichiometry and critically think about the concepts learned during the simulation.
In conclusion, the Stoichiometry PhET Lab is a valuable tool for students to explore and understand the concept of stoichiometry. By engaging in interactive simulations and analyzing the results, students can develop a solid foundation in stoichiometry and strengthen their problem-solving skills in chemistry.
Overview of the PhET Lab

The PhET Lab is an online educational tool that provides interactive simulations to help students learn complex scientific concepts. It covers various topics such as physics, chemistry, biology, and math. One of the popular simulations offered by PhET is the Stoichiometry Lab, which helps students understand the concept of stoichiometry in a fun and engaging way.
The Stoichiometry Lab simulation:
1. Introduction: The lab starts with an introduction to the concept of stoichiometry and its importance in chemical reactions. The simulation provides an overview of the lab environment and the tools available to the students.
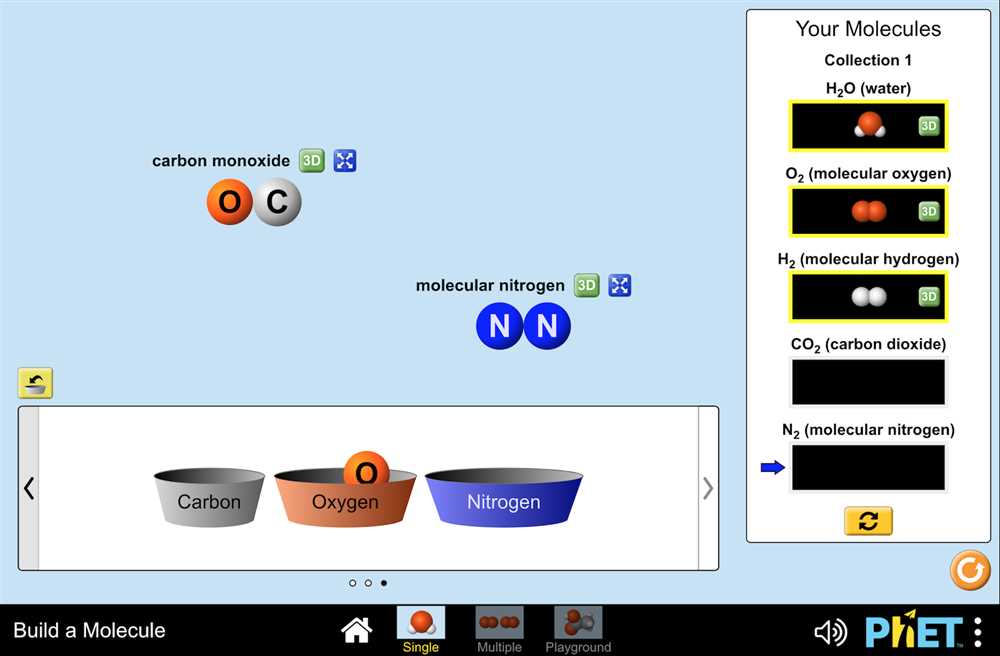
2. Experiment setup: Students are required to set up an experiment by selecting the reactants and their quantities. They can choose from a variety of chemicals and adjust the quantity using sliders or numerical inputs.
3. Observations: Once the experiment is set up, students can observe the reaction taking place in real-time. They can see the changes in the number of atoms and molecules involved in the reaction, as well as the energy changes.
4. Calculations: The simulation provides tools for students to calculate various stoichiometry-related values such as the limiting reactant, the excess reactant, and the theoretical yield. Students can input their calculations and compare them with the simulation’s results.
5. Analysis: After completing the experiment and calculations, students are prompted to analyze the results and answer questions related to the stoichiometric calculations and concepts. This helps reinforce their understanding of the topic.
Overall, the PhET Lab’s Stoichiometry simulation offers an immersive and interactive learning experience for students. It allows them to explore the concepts of stoichiometry firsthand, practice calculations, and gain a deeper understanding of chemical reactions and their quantitative aspects.
Purpose of the PhET Lab
The PhET Lab on stoichiometry is designed to help students understand and apply the principles of stoichiometry, which is a branch of chemistry that deals with the quantification of chemical reactions. By interacting with the virtual lab, students can explore the concept of stoichiometry in a hands-on and interactive manner, allowing them to develop a deeper understanding of the subject.
The main purpose of the PhET Lab is to provide students with a virtual environment where they can manipulate various chemical reactions and observe the resulting changes in the quantities of substances involved. This allows them to see how different reactants and products are related in terms of their ratios and amounts. Through this exploration, students can gain insights into the fundamental concepts of stoichiometry, such as balancing equations, calculating reaction yields, and determining limiting reactants.
The PhET Lab also aims to enhance students’ problem-solving skills and critical thinking abilities. By presenting students with realistic scenarios and challenges, the lab encourages them to analyze and interpret data, make predictions, and draw conclusions based on their observations. Furthermore, the lab provides immediate feedback and supports students in their learning process, helping them to overcome difficulties and reinforce their understanding of stoichiometry.
Equipment and Materials Used in the PhET Lab
The Stoichiometry PhET lab requires several pieces of equipment and materials to conduct the experiments and calculations. The lab is designed to help students understand and practice the concepts of stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions.
1. Digital Balances: Digital balances are used to accurately measure the masses of reactants and products in the lab. These balances have a higher precision and accuracy compared to traditional mechanical balances, allowing for more precise calculations in stoichiometry.
2. Graduated Cylinders: Graduated cylinders are used to measure the volume of liquids in the lab. They have markings on the side that indicate specific volumes, making it easier to measure and record the volumes of reactants and products.
3. Beakers and Erlenmeyer Flasks: Beakers and Erlenmeyer flasks are used to hold and mix the reactants and products in the lab. They come in different sizes and can hold different volumes of liquids, allowing for flexibility in the experiments.
4. Bunsen Burner: A Bunsen burner is used to heat and create reactions in the lab. It provides a consistent and controllable source of flame, allowing for precise control over the temperature and rate of reactions.
5. Chemicals: Various chemicals are used in the lab to simulate different reactions. These chemicals include substances such as acids, bases, and salts; they are carefully measured and mixed according to the stoichiometric ratios to produce the desired reactions.
6. Safety Equipment: Safety equipment, such as gloves, goggles, and lab coats, are essential for the students’ protection during the lab. They help to prevent any accidents or injuries that may occur while handling chemicals or using equipment.
Overall, the equipment and materials used in the PhET lab for stoichiometry play a crucial role in facilitating a hands-on learning experience and reinforcing the fundamental principles of stoichiometry through experimentation.
Methodology Used in Stoichiometry PhET Lab
The Stoichiometry PhET Lab is a virtual lab that allows students to explore and practice stoichiometry calculations in a virtual setting. The lab uses a series of interactive simulations and activities to guide students through the process of balancing chemical equations and determining the amount of reactants and products in a chemical reaction.
One of the main activities in the lab is the Balancing Equations simulation, where students are presented with a chemical equation and must drag and drop the appropriate coefficients to balance the equation. This activity helps students develop a strong foundation in understanding the concept of stoichiometry and the importance of balanced equations in chemical reactions.
Another key component of the Stoichiometry PhET Lab is the Reaction Explorer simulation. In this simulation, students can select different chemical reactions and observe the changes in the quantities of reactants and products as the reaction progresses. This helps students develop an intuitive understanding of stoichiometry and the relationship between the amounts of reactants and products in a chemical reaction.
The lab also includes a variety of practice problems and exercises that allow students to apply the concepts they have learned. These problems require students to calculate the amount of reactants needed, the amount of products formed, and other stoichiometry-related calculations. The virtual nature of the lab allows for immediate feedback and provides an opportunity for students to practice and reinforce their understanding of stoichiometry.
In conclusion, the Stoichiometry PhET Lab uses interactive simulations and activities to engage students in the process of learning and practicing stoichiometry. By providing a virtual environment where students can explore and manipulate chemical reactions, the lab helps students develop a strong foundation in stoichiometry and prepares them for further study in chemistry.