Predicting ionic charges is an essential skill in chemistry, as it allows us to determine the charge of an ion based on the periodic table and the element’s position within it. This skill is crucial in understanding chemical reactions and the formation of compounds.
When predicting ionic charges, it is important to understand the concept of valence electrons. Valence electrons are the electrons found in the outermost energy level of an atom. These electrons are responsible for the atom’s chemical behavior and can be gained, lost, or shared to form chemical bonds.
In order to predict the ionic charge of an element, we can refer to the periodic table. Elements in group 1, also known as the alkali metals, tend to lose one electron and have a charge of +1. Elements in group 2, known as the alkaline earth metals, lose two electrons and have a charge of +2.
On the other hand, elements in groups 15, 16, and 17, known as the nitrogen family, oxygen family, and halogens, respectively, tend to gain electrons and have negative charges. The charge is determined by the number of electrons needed to achieve a stable octet configuration, usually by gaining one, two, or three electrons, resulting in charges of -1, -2, or -3, respectively.
Predicting the ionic charges of transition metals can be more challenging due to their ability to form multiple ions with different charges. In such cases, the ionic charge is determined by the electron configuration of the ion or the number of electrons gained or lost.
In conclusion, predicting ionic charges is a fundamental skill in chemistry that allows us to understand the behavior of elements, compounds, and chemical reactions. By referring to the periodic table and understanding the concept of valence electrons, we can accurately determine the charge of an ion and further explore the fascinating world of chemistry.
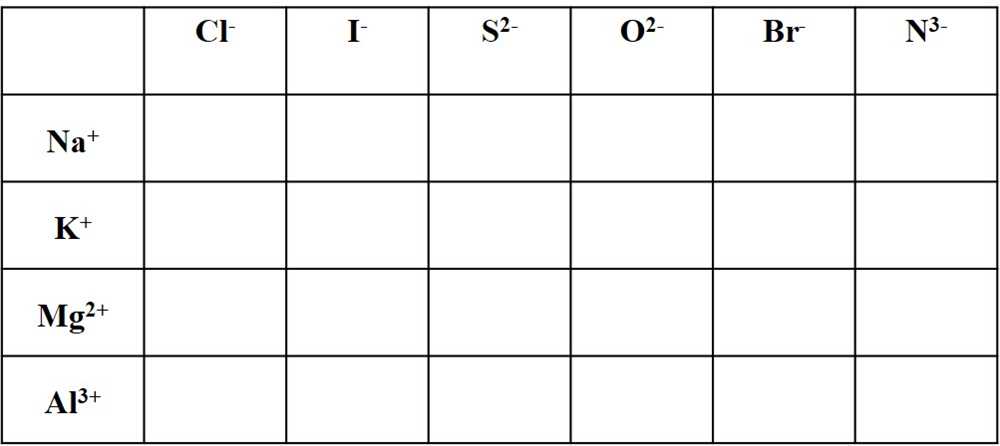
Predicting Ionic Charges Worksheet Answers
When working with ionic compounds, it is important to understand the charges of the ions involved. Predicting the ionic charges of elements can be done by following a set of rules based on the element’s position on the periodic table.
One of the main factors that determines the charge of an ion is the number of valence electrons an element has. Valence electrons are the electrons found in the outermost energy level of an atom. Elements in the same group of the periodic table tend to have the same number of valence electrons, and therefore similar ionic charges.
The following rules can be used to predict the ionic charges of elements:
- Group 1 elements, also known as alkali metals, such as sodium or potassium, have a +1 charge.
- Group 2 elements, also known as alkaline earth metals, such as magnesium or calcium, have a +2 charge.
- Group 13 elements, such as aluminum or boron, have a +3 charge.
- Group 14 elements, such as carbon or silicon, can have various charges, depending on the specific compound.
- Group 15 elements, such as nitrogen or phosphorus, have a -3 charge.
- Group 16 elements, such as oxygen or sulfur, have a -2 charge.
- Group 17 elements, also known as halogens, such as chlorine or fluorine, have a -1 charge.
- Group 18 elements, also known as noble gases, are typically unreactive and have a 0 charge.
By following these rules and knowing the group of an element, it is possible to predict the ionic charge it will form in a compound. However, it is important to note that there are exceptions to these rules, and some elements may have multiple possible charges depending on the specific compound they are part of.
Overall, predicting the ionic charges of elements is an important skill in chemistry, as it allows for the understanding of how ions interact and form compounds.
What are Ionic Charges?

Ionic charges are the electrical charges that result from the gain or loss of electrons by atoms during a chemical reaction. In an ionic bond, atoms transfer electrons to achieve a stable electronic configuration. The atom that loses electrons becomes positively charged, while the atom that gains electrons becomes negatively charged. These charged particles are called ions.
Cations are positively charged ions that are formed when an atom loses one or more electrons. They are usually metals, as metals tend to have low ionization energies, meaning they can easily lose electrons to form positive ions. For example, sodium (Na) has a single electron in its outermost shell, which it readily gives up to become a sodium cation with a charge of +1.
Anions are negatively charged ions that are formed when an atom gains one or more electrons. They are typically nonmetals, as nonmetals have high electron affinities, meaning they can easily gain electrons to form negative ions. For example, chlorine (Cl) has seven electrons in its outermost shell. By gaining one electron, it becomes a chloride anion with a charge of -1.
In some cases, the positive or negative charge on an ion may be greater than one. This occurs when an atom loses or gains multiple electrons. For example, calcium (Ca) can lose two electrons to become a calcium cation with a charge of +2. Oxygen (O) can gain two electrons to become an oxide anion with a charge of -2.
The ionic charges of elements can often be predicted based on their position in the periodic table. Group 1 elements, such as sodium and potassium, typically form cations with a charge of +1. Group 2 elements, such as calcium and magnesium, typically form cations with a charge of +2. On the other hand, group 17 elements, such as chlorine and bromine, typically form anions with a charge of -1.
Being able to predict and understand ionic charges is important in chemistry as it allows us to determine the formulas and names of ionic compounds. It also helps us understand the behavior and reactivity of different elements and compounds.
Why is it Important to Predict Ionic Charges?
Predicting ionic charges is an essential skill in chemistry because it allows us to understand and analyze chemical reactions and reactions between different elements. Ionic charges play a critical role in determining how atoms will bond and interact with one another, forming stable compounds. By being able to predict the charges associated with different elements, we can more effectively determine the formula and properties of compounds.
When chemical reactions occur, the exchange of electrons between atoms results in the formation of bonds. These bonds can be classified as either ionic or covalent, depending on the type of elements involved. In ionic bonding, one or more electrons are transferred from one atom to another, resulting in the formation of ions with distinct charges. To understand and predict the outcome of these reactions, it is crucial to be able to determine the charges of the ions involved.
Predicting ionic charges also allows us to understand the stability and reactivity of different compounds. By knowing the charges of the ions present in a compound, we can determine how they will interact with other substances and participate in chemical reactions. This knowledge is essential in fields such as pharmaceuticals, materials science, and environmental science, where understanding the behavior of different compounds is crucial for designing new drugs, creating new materials, and analyzing environmental processes.
Overall, the ability to predict ionic charges is a fundamental skill in chemistry that allows us to understand the behavior of elements and compounds. It enables us to analyze chemical reactions, determine the formula and properties of compounds, and predict the stability and reactivity of substances. Without this knowledge, our understanding of the chemical world would be limited, hindering advancements in various scientific fields.
Predicting Ionic Charges of Elements in Groups 1 and 2
When it comes to predicting ionic charges of elements in groups 1 and 2, there are certain patterns and trends that can be observed. These elements are known as alkali metals (group 1) and alkaline earth metals (group 2), and they have unique characteristics that determine their ionic charges.
In group 1, alkali metals, such as lithium, sodium, and potassium, tend to lose one electron to achieve a stable electron configuration. This results in a positive charge of +1 for these elements. For example, lithium (Li) loses one electron to become Li+, sodium (Na) loses one electron to become Na+, and potassium (K) loses one electron to become K+.
On the other hand, in group 2, alkaline earth metals, such as beryllium, magnesium, and calcium, tend to lose two electrons to achieve a stable electron configuration. This results in a positive charge of +2 for these elements. For example, beryllium (Be) loses two electrons to become Be2+, magnesium (Mg) loses two electrons to become Mg2+, and calcium (Ca) loses two electrons to become Ca2+.
Overall, the ionic charges of elements in groups 1 and 2 can be predicted based on their tendency to lose electrons and achieve a stable electron configuration. This knowledge is essential in understanding the formation of ionic compounds and the interactions between different elements in chemical reactions.
Predicting Ionic Charges of Elements in Group 7
In chemistry, the Group 7 elements, also known as the halogens, consist of fluorine, chlorine, bromine, iodine, and astatine. These elements occupy the second-to-last column of the periodic table and have unique properties that allow for the prediction of their ionic charges. The ionic charge of an element refers to the number of electrons it gains or loses when forming an ion.
Fluorine, as the first element in Group 7, has a predictable ionic charge of -1. This is because fluorine is highly electronegative and easily gains one electron to achieve a stable octet configuration. Chlorine, the second element, also has a predictable ionic charge of -1 for the same reasons. Similarly, bromine and iodine, the next two elements, have predictable ionic charges of -1 and -1, respectively.
However, astatine, the last element in Group 7, deviates from this trend. Astatine is a highly radioactive element and is not as well-studied as the other halogens. Its ionic charge is predicted to be -1 based on its position in the periodic table, but experimental evidence is limited. Further research is needed to confirm the exact ionic charge of astatine.
It is worth noting that the predictability of the ionic charges of Group 7 elements is due to their tendency to gain one electron and achieve a stable electron configuration. This is known as the octet rule, which states that atoms tend to gain, lose, or share electrons to reach a stable configuration with eight valence electrons. Understanding the predictability of ionic charges is essential for predicting chemical reactions and understanding the behavior of elements within compounds.
- Fluorine: -1
- Chlorine: -1
- Bromine: -1
- Iodine: -1
- Astatine: -1 (predicted)
Predicting Ionic Charges of Transition Metals
Transition metals are a group of elements in the periodic table that have unique properties, including the ability to form ions with different charges. Since transition metals have multiple valence electrons, they can lose different numbers of electrons to form positive ions with varying charges. Predicting the ionic charges of transition metals can be challenging but is essential to understanding their chemical behavior and reactions.
One way to predict the ionic charges of transition metals is by examining their position in the periodic table. Transition metals are located in the d-block, between the s-block and p-block elements. The periodic table can provide insights into their electronic configurations and help determine their potential charges. For example, elements in Group 1 of the periodic table, such as lithium and sodium, tend to lose one electron to form a +1 ion. Transition metals in Group 2, such as magnesium and calcium, often lose two electrons to form a +2 ion.
However, predicting the ionic charges of transition metals becomes more complex as we move further into the d-block. Transition metals can form ions with various charges, often due to their ability to lose different numbers of d-electrons. For example, iron (Fe) can form ions with a +2 or +3 charge, depending on whether it loses two or three electrons. Copper (Cu) can have a +1 or +2 charge, and even a +3 charge in some cases.
It is important to note that predicting the ionic charges of transition metals is not always straightforward and may require additional information, such as the specific chemical reactions or compounds involved. The charge of a transition metal ion can also be influenced by the presence of other elements or ligands in a compound. Overall, understanding the patterns and trends in the periodic table and considering the specific context are key to predicting the ionic charges of transition metals accurately.
Common Exceptions to Predicting Ionic Charges
When predicting the charges of ions in ionic compounds, there are some common exceptions to the usual patterns. These exceptions occur due to the unique characteristics of certain elements or specific compound formations.
One common exception is the transition metals. Transition metals often have multiple possible oxidation states, meaning they can form ions with different charges. This is because transition metals have partially filled d or f orbitals, which allow them to lose different numbers of electrons. For example, iron can have an oxidation state of +2 or +3, and copper can have an oxidation state of +1 or +2.
Another exception is the presence of polyatomic ions. Polyatomic ions are composed of multiple atoms and are treated as a single unit in ionic compounds. Their charges are not always predictable based on the usual patterns for individual elements. For example, the sulfate ion (SO4^2-) has a charge of -2, even though oxygen usually has a charge of -2 and sulfur usually has a charge of +6. It is important to memorize the charges of common polyatomic ions in order to correctly predict the charges of compounds containing them.
Additionally, some elements can have unconventional charges based on specific compound formations. For example, hydrogen can sometimes have a charge of -1 when it is bonded to certain metals or nonmetals. Similarly, elements in unusual combinations, such as oxygen in compounds with fluorine or other highly electronegative elements, can have positive charges.
In summary, while there are general patterns for predicting ionic charges, there are also common exceptions that arise due to the unique characteristics of certain elements or specific compound formations. Being aware of these exceptions and memorizing the charges of common polyatomic ions is crucial for accurately predicting the charges of ions in ionic compounds.