
Understanding and balancing chemical equations is a fundamental skill in chemistry. It allows us to accurately represent and predict the reactions that occur between different substances. However, for many students, balancing chemical equations can be a daunting task. Fortunately, the Balancing Chemical Equations Gizmos provides a valuable resource to help students grasp this important concept.
The Balancing Chemical Equations Gizmos is an online tool that offers a hands-on approach to learning and practicing the art of balancing chemical equations. It provides a variety of interactive activities and simulations that guide students through the process of balancing equations step by step. With its user-friendly interface and comprehensive explanations, the Gizmos answers frequently asked questions and clears any misconceptions students may have about the subject.
One of the key features of the Balancing Chemical Equations Gizmos is its extensive answer key. This answer key provides detailed explanations and solutions to the different exercises and simulations offered by the Gizmos. By referring to the answer key, students can check their work and see where they went wrong, allowing for a deeper understanding of the material and facilitating the learning process.
In conclusion, the Balancing Chemical Equations Gizmos answers offer an invaluable resource for students studying chemistry. With its interactive activities, comprehensive explanations, and extensive answer key, it provides a comprehensive guide to understanding and mastering the art of balancing chemical equations. Whether you are a beginner or an advanced student, the Balancing Chemical Equations Gizmos is a powerful tool that can help you succeed in your chemistry studies.
Balancing Chemical Equations Gizmos Answers
The Balancing Chemical Equations Gizmos is a popular educational tool used to teach students how to balance chemical equations. This interactive online program allows students to practice and develop their skills in balancing equations through a series of guided activities. As students progress through the Gizmos, they are challenged with different scenarios and equations that require them to use their knowledge of chemical formulas and the Law of Conservation of Mass to balance the equations correctly.
One of the most valuable features of the Balancing Chemical Equations Gizmos is the availability of answers and explanations for each activity. This allows students to check their work and understand any mistakes they may have made. By reviewing the answers and explanations, students can identify where they went wrong and learn from their errors. Additionally, the provided answers serve as a useful resource for students who may be struggling to understand certain concepts or equations.
While the Balancing Chemical Equations Gizmos provides answers, it’s important for students to remember that the goal of using this tool is to develop their understanding and proficiency in balancing equations. Simply copying the answers without understanding the underlying principles will hinder their learning progress. Therefore, it is recommended that students use the answers as a reference and compare their own solutions to ensure they have accurately balanced the equations.
In conclusion, the Balancing Chemical Equations Gizmos is an effective tool for students to practice and master the skill of balancing chemical equations. With the availability of answers and explanations, students can check their work and enhance their understanding of the concepts involved. However, it is crucial that students use the answers as a learning tool and actively engage with the equations to develop their problem-solving skills.
What are chemical equations?
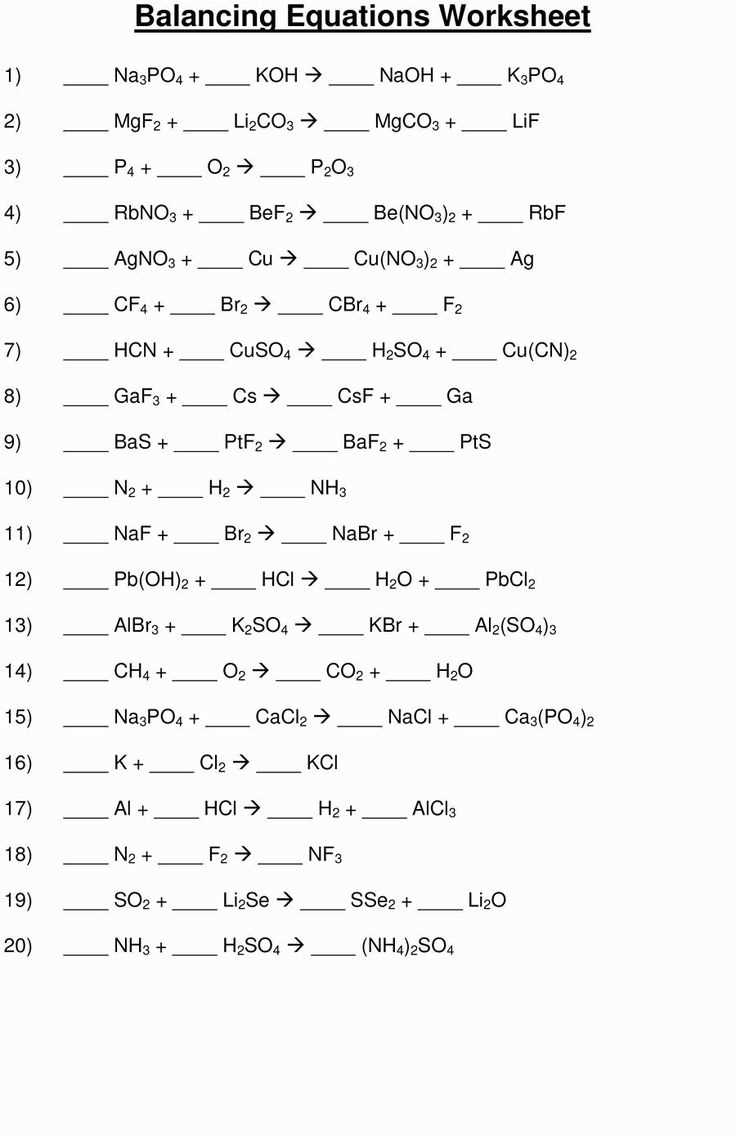
Chemical equations are representations of chemical reactions, which show the relationships between the reactants and products involved. They provide a concise way to describe the changes that occur during a chemical reaction and are written using chemical formulas and symbols.
In a chemical equation, the reactants are written on the left side of the equation and the products on the right side. The reactants are the substances that are present at the beginning of the reaction and the products are the substances that are formed as a result of the reaction.
Example:
The chemical equation for the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O) is:
2H2 + O2 → 2H2O
The numbers in front of the substances are called coefficients and represent the relative amounts of each substance involved in the reaction. In this example, the coefficient “2” indicates that two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water.
Chemical equations must follow the law of conservation of mass, which states that the total mass of the reactants must be equal to the total mass of the products. This is achieved by balancing the equation, ensuring that the number of atoms of each element is the same on both sides of the equation.
Why is it important to balance chemical equations?
Balancing chemical equations is a fundamental aspect of chemistry and is important for several reasons.
Firstly, balancing chemical equations ensures that the law of conservation of mass is obeyed. This law states that matter cannot be created or destroyed in a chemical reaction, only rearranged. By balancing the equation, we are ensuring that the same number of each type of atom is present on both sides of the equation. This allows us to accurately represent the chemical reaction and understand the changes that occur.
Secondly, balanced chemical equations provide us with valuable information about the stoichiometry of a reaction. Stoichiometry is the study of the quantitative relationships between the reactants and the products in a chemical reaction. By balancing the equation, we can determine the mole ratios between the different substances involved. This information is crucial for calculating the amount of reactants needed or the amount of products that can be formed.
Furthermore, balanced chemical equations help chemists in predicting the outcome of a reaction. By knowing the balanced equation and the stoichiometry, chemists can determine how much reactant is needed to produce a specific amount of product or vice versa. This information is essential for designing and optimizing chemical processes.
In summary, balancing chemical equations is important because it ensures the conservation of mass, provides information about stoichiometry, and allows for predictions and calculations in chemical reactions. It is a fundamental skill that all chemists must possess, as it forms the basis for further understanding and research in the field of chemistry.
Balancing chemical equations with Gizmos
Balancing chemical equations is an important skill in chemistry that allows us to understand the relationship between reactants and products in a chemical reaction. It involves making sure that the number of atoms of each element is the same on both sides of the equation. One effective tool for learning and practicing this skill is the Balancing Chemical Equations Gizmo.
The Gizmo provides an interactive platform where students can experiment with different chemical reactions and balance the equations step by step. This hands-on approach allows students to visualize the process and develop an intuitive understanding of how atoms and molecules interact in a reaction. The Gizmo also provides real-time feedback, highlighting any errors in balancing the equation and guiding students towards the correct solution.
With the Balancing Chemical Equations Gizmo, students can explore a variety of reactions, from simple single-element reactions to more complex multi-element reactions. They can adjust the coefficients of the reactants and products and observe how these changes affect the balance of the equation. This interactive experience helps students grasp the concept of stoichiometry and gain a deeper understanding of the conservation of mass in chemical reactions.
In addition to helping students develop their problem-solving skills and critical thinking abilities, the Balancing Chemical Equations Gizmo also promotes collaboration and teamwork. Students can work together in pairs or small groups to discuss and analyze the reactions, share their ideas, and find the best way to balance the equations. This collaborative learning environment fosters a sense of engagement and excitement, making the process of balancing chemical equations more enjoyable and memorable.
Overall, the Balancing Chemical Equations Gizmo is a valuable tool for teaching and learning chemistry. It provides an interactive and visual approach to balancing chemical equations, helping students develop a deep understanding of the principles behind chemical reactions. By using this Gizmo, students can gain the necessary skills and confidence to tackle more complex problems in chemistry and excel in their academic endeavors.
Step-by-step guide to balancing chemical equations with Gizmos
Chemical equations are used to represent the reactions between different substances, and balancing these equations is a crucial skill in chemistry. The Gizmos tool provides an interactive way to practice balancing chemical equations and understand the underlying concepts.
1. Select the chemical equation Gizmo: Start by accessing the Gizmos platform and navigating to the chemical equations section. Look for the specific Gizmo that focuses on balancing chemical equations. Click on it to open the interactive tool.
2. Understand the equation: Once the Gizmo is open, familiarize yourself with the chemical equation that needs to be balanced. Take note of the reactants on the left side and the products on the right side. Identify the elements and compounds involved, as well as their coefficients.
3. Begin balancing: Start by adjusting the coefficients of the reactants and products in order to make the number of atoms on both sides of the equation equal. Begin with the elements that appear in the fewest compounds, as this can simplify the balancing process.
4. Use the Gizmo tools: The Gizmos platform provides various tools to help with balancing chemical equations. You can click on the coefficients to increase or decrease their values, or use the + and – buttons to adjust them. Take note of the changes in the number of atoms on each side of the equation as you make adjustments.
5. Validate and refine: After making adjustments, check if the equation is balanced. Ensure that the number of atoms is the same on both sides of the equation. If the equation is still unbalanced, continue making changes until it is balanced.
6. Review and learn: After successfully balancing the chemical equation, take a moment to review the changes you made and understand the reasoning behind them. Pay attention to the principles of conservation of mass and the law of definite proportions.
7. Practice with different equations: Once you are comfortable with one equation, practice balancing different chemical equations using the Gizmo. This will help reinforce your understanding and improve your skills in balancing equations.
By following this step-by-step guide and utilizing the Gizmos tool, you can enhance your ability to balance chemical equations and gain a deeper understanding of chemical reactions.
Common challenges in balancing chemical equations
When it comes to balancing chemical equations, there are several common challenges that students may face. One of the main challenges is understanding the concept of conservation of mass. The principle states that in a chemical reaction, the total mass of the reactants must be equal to the total mass of the products. This means that the number of atoms of each element must be the same on both sides of the equation. Students often struggle with this concept and may find it difficult to balance equations correctly.
Another common challenge is identifying the correct coefficients for each compound or element in the equation. This requires a deep understanding of stoichiometry and the ability to determine the molar ratios between reactants and products. Sometimes, students may make errors in identifying the correct ratios, leading to imbalanced equations. It is important for students to practice and develop their skills in stoichiometry in order to overcome this challenge.
Additionally, some chemical equations may involve complex compounds or reactions that are not easily balanced. These equations may require the use of advanced techniques, such as simultaneous equations or trial and error, to find the correct coefficients. Students may find these types of equations particularly challenging and may need to seek additional resources or guidance to successfully balance them.
In conclusion, balancing chemical equations can be a challenging task for students. Understanding the concept of conservation of mass, accurately identifying coefficients, and dealing with complex equations are some of the common challenges that students may face. With practice and a solid understanding of the underlying principles, however, students can overcome these challenges and become proficient in balancing chemical equations.
Examples of balanced chemical equations using Gizmos
One of the helpful tools for balancing chemical equations is the Gizmos simulation software. It allows users to practice and experiment with different chemical reactions and their corresponding equations. Here are a few examples of balanced chemical equations using Gizmos:
Example 1: Combustion of methane
One common reaction is the combustion of methane (CH4). With Gizmos, you can balance this equation by adding coefficients to the reactants and products until the number of atoms on each side is equal. The balanced equation for the combustion of methane is:
CH4 + 2O2 → CO2 + 2H2O
Example 2: Formation of water
Another example is the formation of water (H2O) from its elements hydrogen (H2) and oxygen (O2). By using Gizmos, you can determine the correct coefficients to balance the equation:
2H2 + O2 → 2H2O
Example 3: Sodium chloride precipitation reaction
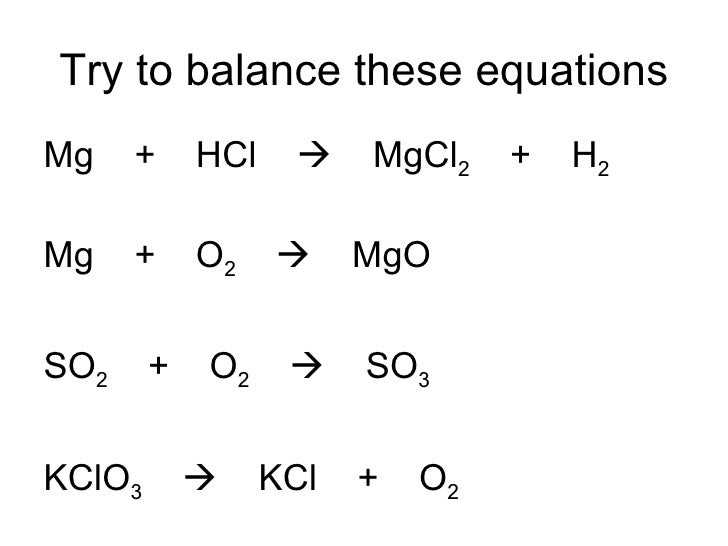
Gizmos can also be used to balance precipitation reactions, such as the formation of sodium chloride (NaCl) from sodium ions (Na+) and chloride ions (Cl-). The balanced equation for this reaction is:
Na+ + Cl- → NaCl
These are just a few examples of the balanced chemical equations that can be achieved using Gizmos. With its interactive interface and step-by-step guidance, Gizmos can help students and researchers alike to practice and master the skill of balancing chemical equations. It provides a hands-on approach to understanding the fundamental principles of chemical reactions.