In Chapter 5 of a textbook on chemistry, the focus is on understanding electrons in atoms. This chapter delves into the structure of atoms, the arrangement of electrons, and how the movement of electrons within an atom affects its overall properties. Understanding this fundamental concept is crucial for comprehending the behavior of matter and the chemical reactions that occur.
By exploring the answer key for Chapter 5, students can check their understanding of the material and assess their knowledge. The answer key acts as a valuable resource to verify whether one has grasped the concepts correctly and completed the assigned exercises accurately. It also helps students identify any areas of weakness or misunderstanding, enabling them to seek further clarification and reinforcement.
This chapter covers various topics, including the Bohr model of the atom, electron configurations, and quantum numbers. Through the answer key, students can verify their ability to apply these concepts correctly. The questions and problems in the exercises are designed to challenge students and test their understanding of the inner workings of atoms.
Chapter 5.1 Electrons in Atoms Answer Key
In Chapter 5.1 of the textbook, we explore the concept of electrons in atoms and their arrangement within the atom. This answer key provides a comprehensive understanding of the various aspects covered in this chapter.
The chapter begins by introducing the concept of electromagnetic radiation and its relationship to the behavior of electrons. It discusses the wave-particle duality of electrons and explains how their behavior can be described using both wave and particle models.
- Question 1: What is the relationship between electromagnetic radiation and electrons?
- Answer: Electromagnetic radiation, such as light, consists of waves that contain both electric and magnetic fields. Electrons can interact with these waves, exhibiting both wave-like and particle-like behavior.
Next, the chapter discusses the different atomic models proposed by scientists throughout history, including the Bohr model and the quantum mechanical model. It explains how these models provide a framework for understanding the arrangement of electrons within an atom and their energy levels.
- Question 2: What are the main differences between the Bohr model and the quantum mechanical model?
- Answer: The Bohr model is a simplified model that describes electrons as orbiting the nucleus in specific energy levels, similar to planets orbiting the sun. The quantum mechanical model, on the other hand, describes electrons as existing in probability regions called orbitals, with specific energy levels and sublevels.
The chapter also delves into the concept of electron configuration and the rules governing the filling of electron orbitals. It explains the Aufbau principle, Hund’s rule, and the Pauli exclusion principle, which determine the order and arrangement of electrons within an atom.
- Question 3: What is the significance of the Aufbau principle, Hund’s rule, and the Pauli exclusion principle?
- Answer: The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy levels. Hund’s rule states that electrons first occupy separate orbitals within a sublevel before pairing up. The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers.
In conclusion, Chapter 5.1 provides a comprehensive understanding of electrons in atoms, including their behavior as both waves and particles, the different atomic models proposed throughout history, and the rules governing electron configuration. This answer key serves as a valuable resource for mastering the concepts covered in this chapter.
Understanding Electrons in Atoms
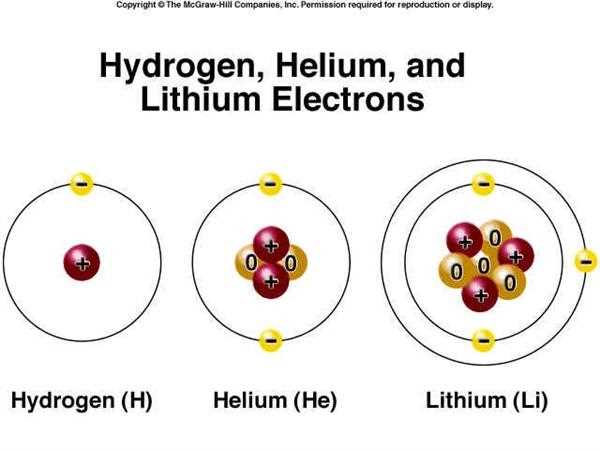
Electrons are fundamental particles that orbit the nucleus of an atom. They have a negative charge and play a crucial role in determining the properties and behavior of elements. Understanding electrons in atoms is essential for comprehending concepts such as atomic structure, chemical bonding, and the periodic table.
One key aspect of electrons in atoms is their arrangement in energy levels or shells. These energy levels, labeled as K, L, M, and so on, can accommodate a specific number of electrons. The closer an energy level is to the nucleus, the lower its energy and the lower the number of electrons it can hold. For example, the first energy level (K) can hold a maximum of 2 electrons, while the second (L) can hold up to 8 electrons.
The distribution of electrons in energy levels follows the Aufbau principle, which states that electrons fill the lowest energy levels first before occupying higher energy levels. This principle helps explain the periodicity of the elements in the periodic table. Each row in the periodic table represents a period, and the position of an element in a period corresponds to the energy level of its outermost electrons.
Electrons also exhibit a phenomenon known as electron configuration, which describes the specific arrangement of electrons in an atom. Electron configurations are represented using a combination of numbers, letters, and superscripts called orbital notations. These notations provide valuable information about the distribution of electrons in different energy levels and sublevels, such as the s, p, d, and f orbitals.
The study of electrons in atoms is crucial for understanding various phenomena in chemistry, ranging from the behavior of elements in reactions to the formation of chemical bonds. It allows scientists to predict and explain the properties and reactivity of substances, providing a foundation for the development of new materials and the advancement of technology.
The Role of Electrons in Atomic Structure
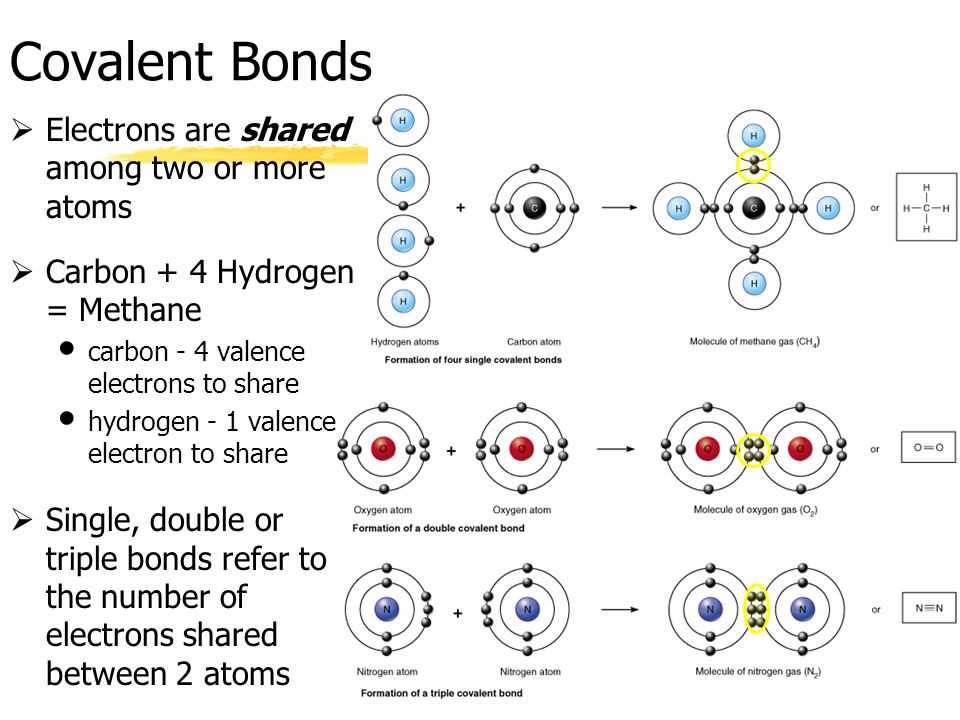
Electrons play a crucial role in the structure of atoms. They are subatomic particles that orbit around the nucleus of an atom, forming a cloud-like electron cloud. The arrangement and behavior of electrons determine the properties and characteristics of each element.
Electron Configuration and Energy Levels: Electrons occupy specific energy levels or orbitals around the nucleus. These energy levels are represented by different shells, with the closest shell to the nucleus having the lowest energy level. Within each shell, there are subshells (s, p, d, and f) that further classify the location of electrons. The electron configuration of an atom describes the arrangement of electrons in these energy levels and subshells.
Valence Electrons and Chemical Behavior: The outermost shell of an atom, known as the valence shell, determines the element’s chemical properties. The electrons in this shell are called valence electrons. Valence electrons participate in chemical reactions and bonding with other atoms. The number of valence electrons influences an atom’s reactivity and the types of compounds it can form.
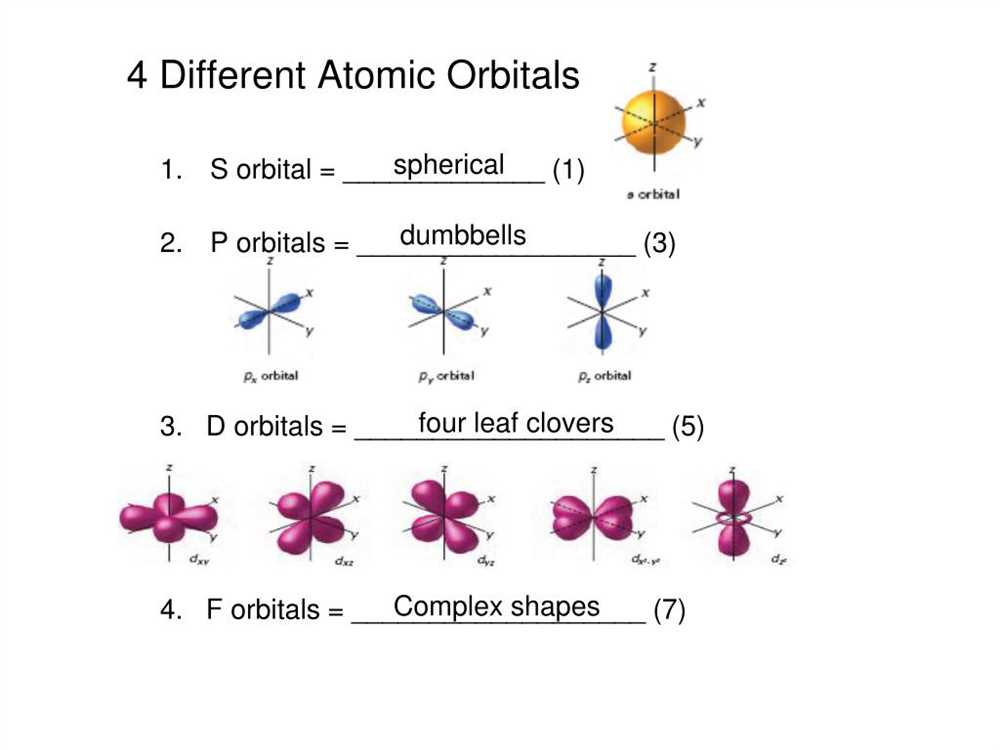
Orbital Shapes and Electron Distribution: Electrons occupy specific orbitals within the energy levels. These orbitals have distinct shapes, such as spherical for s orbitals, dumbbell-shaped for p orbitals, and more complex shapes for d and f orbitals. Electrons are distributed in these orbitals according to the aufbau principle, which states that electrons fill the lowest energy orbitals first before occupying higher energy levels.
Electron Configurations and the Periodic Table: The electron configurations of elements follow a pattern based on the periodic table. The periodic table is organized in such a way that elements with similar electron configurations and properties are grouped together. Understanding the electron configurations of elements helps predict their chemical behavior and reactivity.
In conclusion, electrons play a fundamental role in determining the structure, properties, and behavior of atoms. Their arrangement within energy levels and orbitals, as well as their distribution and participation in chemical reactions, contribute to the complexity and diversity of the elements in the periodic table.
Electron Configuration and Energy Levels
The electron configuration of an atom refers to the arrangement of electrons in its various energy levels. Energy levels are regions around the nucleus where electrons are likely to be found. Each energy level can hold a specific number of electrons, and these levels are filled in a specific order according to the Aufbau principle.
The Aufbau principle states that electrons fill the lowest energy level first before moving on to higher energy levels. The energy levels are designated by the principal quantum number (n), with the first energy level being closest to the nucleus (n = 1) and subsequent energy levels increasing in distance from the nucleus (n = 2, 3, 4, and so on).
The energy levels themselves are composed of sublevels, which are designated by the subshell letter (s, p, d, f) followed by the principal quantum number (n). For example, the first energy level contains only an s sublevel (1s), while the second energy level contains an s and a p sublevel (2s and 2p). Each sublevel can hold a specific number of electrons, with the s sublevel holding 2 electrons, the p sublevel holding 6 electrons, the d sublevel holding 10 electrons, and the f sublevel holding 14 electrons.
Understanding the electron configuration and energy levels is important in determining the chemical properties and behavior of an atom. The number and arrangement of electrons in an atom’s energy levels can help predict its reactivity and ability to form chemical bonds. Furthermore, it provides insights into the electronic structure and stability of an atom, which is crucial in understanding its behavior in chemical reactions.
Orbitals and Quantum Numbers
In the study of electrons in atoms, orbitals and quantum numbers play a crucial role in describing the behavior and location of electrons within an atom.
A quantum number is a set of values that are used to describe the characteristics of an electron in an atom. There are four quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms).
The principal quantum number (n) indicates the energy level of an electron in an atom. It can have any positive integer value, such as 1, 2, 3, and so on. The higher the value of n, the higher the energy level of the electron.
The azimuthal quantum number (l) gives information about the shape of the orbital. It can have values ranging from 0 to n-1. The value of l determines the shape of the orbital, with l = 0 representing an s orbital, l = 1 representing a p orbital, l = 2 representing a d orbital, and so on.
The magnetic quantum number (ml) specifies the orientation of the orbital in space. It can have values ranging from -l to +l, including 0. For example, a p orbital can have three different orientations: ml = -1, 0, and 1.
The spin quantum number (ms) describes the direction of the electron spin. It can have values of +1/2 or -1/2.
By considering the values of these quantum numbers, scientists can determine the possible orbitals that electrons can occupy in an atom and understand their behavior within the atom.
The Pauli Exclusion Principle and Hund’s Rule
The Pauli Exclusion Principle and Hund’s Rule are fundamental principles in quantum mechanics that govern the behavior of electrons in atoms. These principles dictate how electrons occupy atomic orbitals and determine the overall electronic configuration of an atom.
The Pauli Exclusion Principle, stated by Wolfgang Pauli in 1925, states that no two electrons in an atom can have the same set of quantum numbers. This means that each electron in an atom must have a unique combination of the four quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s).
As a result of the Pauli Exclusion Principle, each atomic orbital can accommodate a maximum of two electrons with opposite spins. This means that if one electron occupies a certain orbital, the next electron must have an opposite spin and occupy the same orbital. This principle ensures that electrons in an atom are properly distributed among the available orbitals.
Hund’s Rule, formulated by Friedrich Hund in 1927, further elaborates on the distribution of electrons in atomic orbitals. Hund’s Rule states that when multiple orbitals of the same energy level (degenerate orbitals) are available, electrons will occupy separate orbitals with parallel spins before pairing up. This principle maximizes the total electron spin and leads to greater stability in the atom.
In summary, the Pauli Exclusion Principle and Hund’s Rule guide the distribution of electrons in atomic orbitals, ensuring that each electron has a unique set of quantum numbers and orbitals are filled in a specific manner. These principles play a crucial role in understanding the electronic structure of atoms and explaining various properties and behaviors of elements.
Noble Gas Notation and Abbreviated Electron Configurations
The electron configuration of an atom refers to the arrangement of its electrons in various energy levels and orbitals. In order to simplify and streamline the writing of electron configurations, the use of noble gas notation and abbreviated electron configurations can be employed.
Noble gas notation involves representing the electron configuration of an atom by using the symbol of the noble gas element that precedes it in the periodic table, followed by the remaining electron configuration. This helps to shorten the configuration by indicating that the noble gas’s electron arrangement is already included. For example, the electron configuration of chlorine (Cl) can be written as [Ne] 3s2 3p5, where [Ne] represents the electron configuration of neon (Ne).
Abbreviated electron configurations further simplify the notation by condensing the electron configuration of an atom using the noble gas notation and then only specifying the differences from the noble gas configuration. For example, the abbreviated electron configuration of chlorine can be written as [Ne] 3s2 3p5 or simply as [Ne] 3p5, as the 3s2 electrons are already included in the noble gas notation.
The use of noble gas notation and abbreviated electron configurations allows for a more concise representation of the electron arrangement in an atom, making it easier to comprehend and compare electron configurations across different elements in the periodic table.