
Understanding electron configuration is crucial in studying the behavior and properties of chemical elements. The electron configuration of an atom describes how its electrons are arranged in different energy levels or orbitals. It plays a significant role in determining the chemical reactivity and bonding of elements. To help students practice and master electron configuration, a worksheet with answer key is provided to aid in their learning process.
The electron configuration worksheet contains a series of questions that require students to determine the electron configuration of various elements. It provides them with the chemical symbol of the element and asks them to write the arrangement of electrons in respective orbitals. These questions are designed to test the students’ understanding of the periodic table and the rules governing electron arrangement.
The answer key provided alongside the worksheet allows students to check their work and verify if they have correctly determined the electron configuration. It serves as a useful tool for self-assessment and helps students identify any mistakes or areas they need to review. By comparing their answers with the answer key, students can gain confidence in their knowledge of electron configuration and improve their understanding of the topic.
Overall, the electron configuration worksheet with answer key is an invaluable resource for students studying chemistry. It provides them with a practical means of practicing and assessing their understanding of electron configuration, allowing them to strengthen their knowledge and skills in this fundamental aspect of chemistry.
Electron Configuration Worksheet Answer Key
Electron configuration is an important concept in understanding the arrangement of electrons in an atom. It helps us predict the chemical and physical properties of elements and their ions. An electron configuration worksheet is a tool commonly used to practice and test knowledge of electron configurations. The answer key is a crucial component of the worksheet as it provides the correct electron configurations for the given elements.
The electron configuration worksheet answer key typically includes the element symbol or name, followed by the electron configuration notation. The electron configuration notation consists of a series of letters and numbers that represent the specific orbitals and the number of electrons in each orbital. It may also include additional information such as the valence electron count or the energy level of the electron.
| Element | Electron Configuration |
|---|---|
| Lithium (Li) | 1s2 2s1 |
| Oxygen (O) | 1s2 2s2 2p4 |
| Iron (Fe) | 1s2 2s2 2p6 3s2 3p6 4s2 3d6 |
It is important to note that the electron configuration can vary depending on the ionization state of the element. For example, the electron configuration of oxygen in its neutral state is 1s2 2s2 2p4, but when it forms an ion with a charge of -2, the electron configuration becomes 1s2 2s2 2p6.
By using the electron configuration worksheet answer key, students can check their answers and ensure their understanding of electron configuration. It serves as a valuable tool for practicing and reinforcing the principles of electron configuration in chemistry.
Understanding Electron Configuration

Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons in an atom or molecule. It provides valuable insights into an atom’s stability, reactivity, and chemical properties. By understanding electron configuration, we can predict an atom’s behavior and its interactions with other atoms.
The electron configuration of an atom is written using a series of numbers and letters to represent the energy levels (or shells) and orbitals occupied by electrons. The numbers represent the principal quantum number (n), which determines the energy level of the electrons. The letters (s, p, d, f) represent the subshells or orbitals within each energy level.
The electron configuration follows specific rules, such as the Aufbau principle, Pauli exclusion principle, and Hund’s rule. The Aufbau principle states that electrons fill the lowest energy levels first before moving to higher energy levels. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spin. Hund’s rule states that electrons will fill each orbital in a subshell before pairing up.
To determine the electron configuration of an atom, we follow the periodic table and fill orbitals in a specific order. The 1s orbital is filled first, followed by the 2s and then the 2p orbitals. This pattern continues until all the electrons are accounted for. The electron configuration can also be represented using shorthand notations such as noble gas configurations.
| Atom | Electron Configuration |
|---|---|
| Lithium (Li) | 1s2 2s1 |
| Oxygen (O) | 1s2 2s2 2p4 |
| Iron (Fe) | [Ar] 3d6 4s2 |
Overall, electron configuration is a crucial concept in understanding the properties and behaviors of atoms. It allows us to predict how an atom will bond with other atoms and how it will interact in chemical reactions. By mastering electron configuration, we gain a deeper understanding of the building blocks of matter and their behavior.
The Importance of Electron Configuration
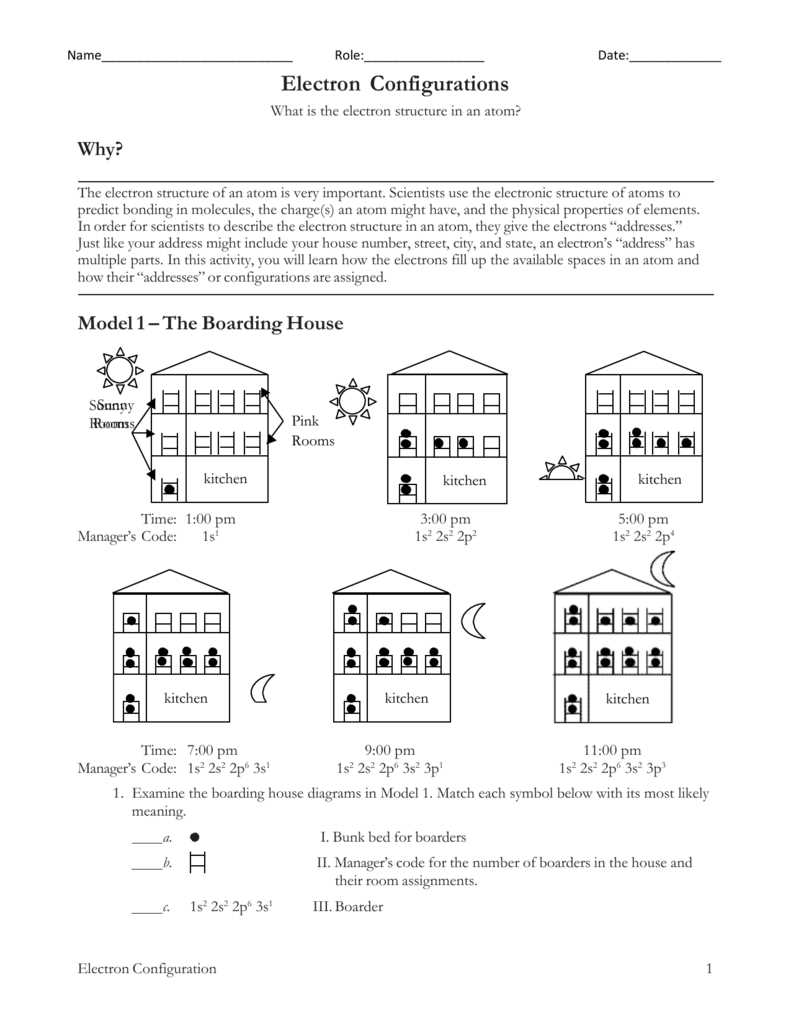
Understanding electron configuration is crucial for studying and predicting the behavior of atoms and molecules. Electron configuration refers to the arrangement of electrons within an atom or a molecule’s orbitals. It provides valuable information about an element’s chemical properties, reactivity, and bonding behavior. By knowing the distribution of electrons in the different energy levels and orbitals, scientists can make accurate predictions about an element’s behavior and its interactions with other elements.
One key aspect of electron configuration is the concept of energy levels and sublevels. Electrons occupy specific energy levels, starting from the lowest energy level and then filling up the higher levels according to specific rules. Each energy level is further divided into sublevels, or orbitals, which can hold a specific number of electrons. The way in which electrons are arranged within these energy levels and sublevels determines the element’s chemical properties and its reactivity.
The electron configuration helps explain why some elements are more reactive than others and why some elements readily form compounds while others do not. For example, elements with similar electron configurations tend to have similar chemical properties and react in similar ways. This is because their electron configurations determine the stability of their outermost (valence) electrons. Elements with a stable electron configuration tend to be less reactive, while those with an incomplete or unstable electron configuration are more likely to form compounds and participate in chemical reactions.
Furthermore, electron configuration also plays a significant role in understanding the periodic trends observed in the periodic table. The organization of elements in the periodic table is based on the patterns and trends in their electron configurations. By analyzing the electron configurations of different elements, scientists can determine their position in the periodic table, predict their reactivity and bonding behavior, and make connections between different elements.
In conclusion, electron configuration is a fundamental concept in chemistry that provides crucial information about the behavior and properties of elements. It helps explain the reactivity of elements, their ability to form compounds, and their position in the periodic table. By understanding electron configuration, scientists can make accurate predictions and explanations about the behavior of atoms and molecules, advancing our knowledge and understanding of the natural world.
Rules for Writing Electron Configurations
Writing electron configurations is an essential skill in chemistry, as it allows us to understand the arrangement of electrons in an atom. By following a set of rules, we can determine the correct electron configuration for any element.
1. Aufbau Principle:
The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. This means that when writing an electron configuration, we start with the lowest energy level and gradually fill up the higher energy levels.
2. Pauli Exclusion Principle:
The Pauli exclusion principle states that each electron in an atom must have a unique set of quantum numbers, including its spin. This means that each orbital can only hold a maximum of two electrons with opposite spins.
3. Hund’s Rule:
Hund’s rule states that when filling degenerate orbitals (orbitals with the same energy), electrons will occupy different orbitals with the same spin before pairing up. This is known as the “spread them out, then pair them up” rule.
By applying these rules, we can accurately write the electron configurations for all elements in the periodic table. This allows us to understand the organization of electrons and their influence on an atom’s chemical behavior. It is an essential tool for chemists in predicting and explaining various chemical properties and reactions.
The Aufbau Principle
The Aufbau Principle is a fundamental concept in understanding electron configurations. It states that electrons fill orbitals in an atom in order of increasing energy. This means that electrons will occupy the lowest energy orbital available before moving to higher energy orbitals.
This principle is based on the idea that electrons are attracted to the positively charged nucleus of an atom and are therefore more stable when they occupy orbitals closer to the nucleus. As electrons fill the orbitals in an atom, they will follow a specific pattern, known as the Aufbau diagram or the “building up” principle.
According to the Aufbau Principle, the 1s orbital will be filled first, followed by the 2s and then the 2p orbitals. The specific order in which the orbitals are filled is determined by the energy levels and sublevels of the orbitals. For example, the 2p orbitals are of higher energy than the 2s orbital, so they are filled after the 2s orbital.
The Aufbau Principle is essential for predicting and understanding the arrangement of electrons in an atom. It allows chemists to determine the electron configuration of an element and helps explain the periodic trends observed on the periodic table. By following the Aufbau Principle, chemists can understand why certain elements have similar properties and how the arrangement of electrons affects an element’s behavior.
Hund’s Rule

Hund’s Rule is a fundamental principle in atomic physics that helps determine the electron configuration of atoms. It states that when filling up multiple orbitals of the same energy level, electrons will first occupy each orbital with the same spin before pairing up.
This rule is based on the principle of electron spin, which states that electrons can have two possible spin states: spin-up (+1/2) or spin-down (-1/2). According to Hund’s Rule, electrons prefer to occupy separate orbitals with the same spin in order to minimize the repulsion between electrons.
For example, let’s consider the electron configuration of nitrogen (N) with an atomic number of 7. Nitrogen has 7 electrons, and its electron configuration can be written as 1s2 2s2 2p3. In the 2p sublevel, there are three orbitals available: 2px, 2py, and 2pz. According to Hund’s Rule, the first two electrons will occupy separate orbitals with the same spin (for example, 2px↑ and 2py↑), and the remaining three electrons will fill up the remaining orbitals (2px↓, 2py↓, and 2pz↓).
In summary, Hund’s Rule helps determine the distribution of electrons in different orbitals of the same energy level. It helps establish the electron configuration of atoms and plays a crucial role in understanding the behavior and properties of elements.
Pauli Exclusion Principle
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics that states that no two identical fermions can occupy the same quantum state simultaneously. This principle, which was formulated by Austrian physicist Wolfgang Pauli in 1925, has significant implications for the electronic structure and behavior of atoms.
According to the Pauli Exclusion Principle, electrons in an atom must occupy different quantum states, characterized by different combinations of quantum numbers such as the principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number. This principle ensures that the electrons in an atom are distributed in various energy levels and subshells, leading to the hierarchical arrangement of electron shells and subshells in the periodic table.
Key points about the Pauli Exclusion Principle:
- The Pauli Exclusion Principle applies only to fermions, which include electrons, protons, and neutrons.
- It restricts the number of electrons that can occupy a particular energy level and orbital in an atom.
- Each electron in an atom is described by a unique set of quantum numbers, which determines its quantum state.
- The principle allows for the formation of chemical bonds and the stability of matter.
- The Pauli Exclusion Principle is essential in explaining the behavior of electrons in atoms, including their arrangement in orbitals and their response to external stimuli.
The Pauli Exclusion Principle plays a crucial role in understanding the electronic configuration of atoms and the properties of materials. It explains why certain elements are more likely to form stable compounds and why electrons exhibit specific patterns of behavior in atoms and molecules. This principle has had a profound impact on the development of quantum mechanics and continues to be a fundamental concept in modern physics.
Electron Configuration Notation
The electron configuration notation is a way of representing the arrangement of electrons in an atom. It is based on the principle that electrons occupy specific energy levels, or shells, within an atom. Each energy level can hold a maximum number of electrons, and the notation represents these electrons as superscripts following the symbol of the element.
The electron configuration notation follows the format of “shell number – subshell letter – number of electrons”. The shell number represents the energy level, ranging from 1 to 7, where 1 is the lowest energy level and 7 is the highest. The subshell letter represents the type of subshell, such as s, p, d, or f. The number of electrons represents the number of electrons in that particular subshell.
For example, the electron configuration of helium is 1s2. This means that helium has two electrons in its first energy level (shell 1) in the s subshell. Similarly, the electron configuration of sodium is 1s2 2s2 2p6 3s1. This means that sodium has two electrons in its first energy level (shell 1), eight electrons in its second energy level (shells 2 and 3), and one electron in its third energy level (shell 3).
Electron configuration notation is important because it provides information about the electronic structure of an atom. It can help determine the chemical behavior and reactivity of an element, as well as its position in the periodic table. Understanding the electron configuration notation is essential in studying the properties and behavior of atoms and molecules.