In the study of chemistry, the concept of atoms is fundamental. Atoms are the building blocks of matter, and understanding their properties and behavior is crucial in explaining the behavior of substances. A worksheet on the history of atoms can provide students with a comprehensive understanding of how our knowledge of atoms has evolved over time.
The history of atoms traces back to ancient civilizations, where philosophers and scientists pondered the nature of matter. Greek philosophers like Democritus proposed the idea that matter was composed of indivisible particles called atoms. However, it wasn’t until much later that experimental evidence supported this idea.
In the 19th century, John Dalton formulated the atomic theory, which stated that all matter is composed of tiny, indivisible particles called atoms. Dalton’s theory laid the groundwork for our modern understanding of atoms and their behavior. However, as technology advanced, scientists discovered that atoms were not as simple as Dalton initially thought.
The discovery of subatomic particles like electrons, protons, and neutrons added complexity to the model of the atom. J.J. Thomson’s experiments with cathode rays led to the identification of electrons, while Ernest Rutherford’s gold foil experiment provided evidence for the existence of a tiny, dense nucleus in the center of an atom. These discoveries revolutionized our understanding of atoms and led to the development of new models to describe their structure and behavior.
The concept of atoms
Atoms are the basic building blocks of matter. They are the smallest particles that make up all the elements in the periodic table. The concept of atoms has been around for thousands of years, with ancient philosophers like Democritus proposing that there must be indivisible particles that make up everything in the world.
In the late 18th century, John Dalton formalized the concept of atoms as the fundamental units of matter. He proposed that atoms are indivisible and indestructible, and that different elements are composed of different types of atoms. This idea laid the foundation for modern atomic theory and our understanding of chemistry.
Key concepts related to atoms:
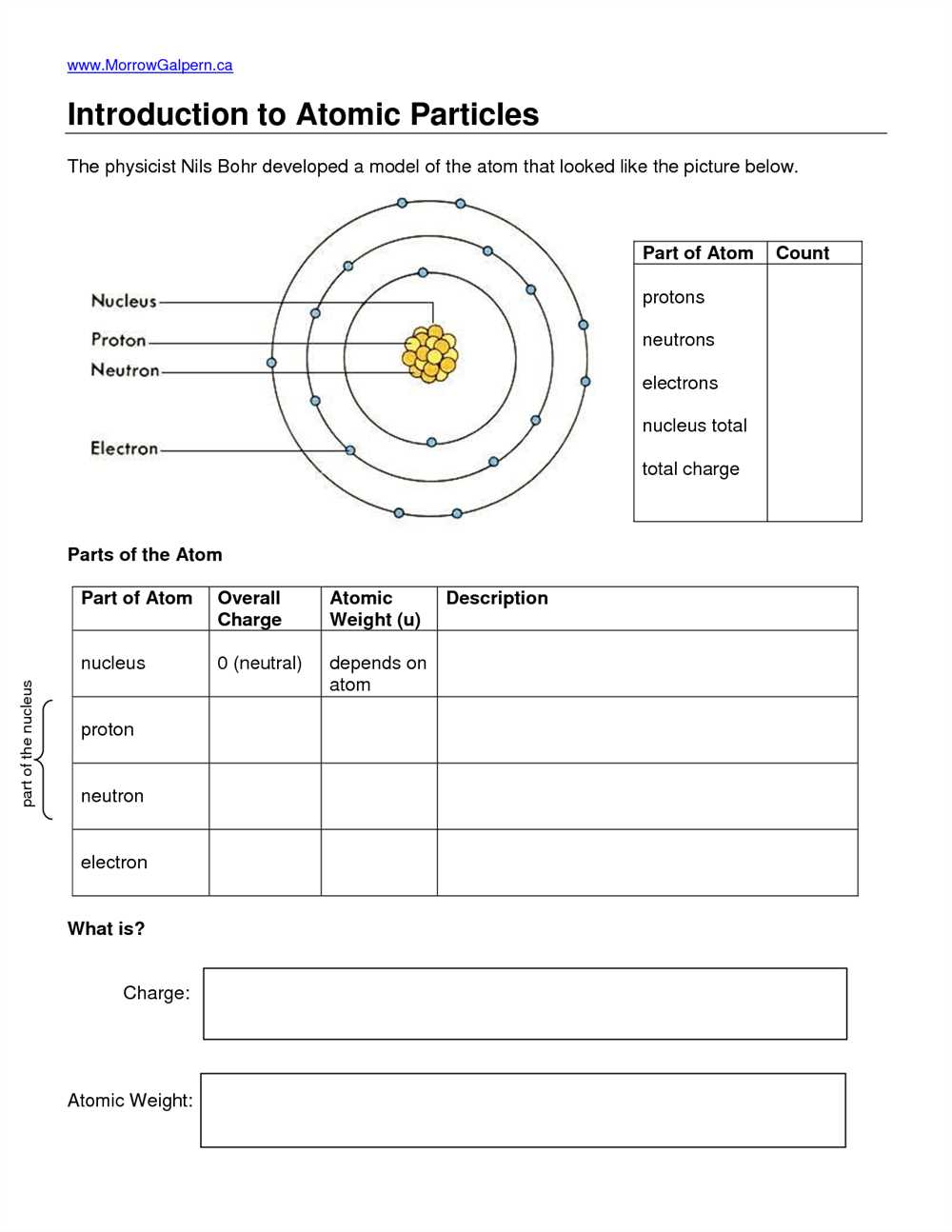
- Atomic structure: Every atom consists of a nucleus, which contains protons and neutrons, surrounded by electrons that occupy energy levels.
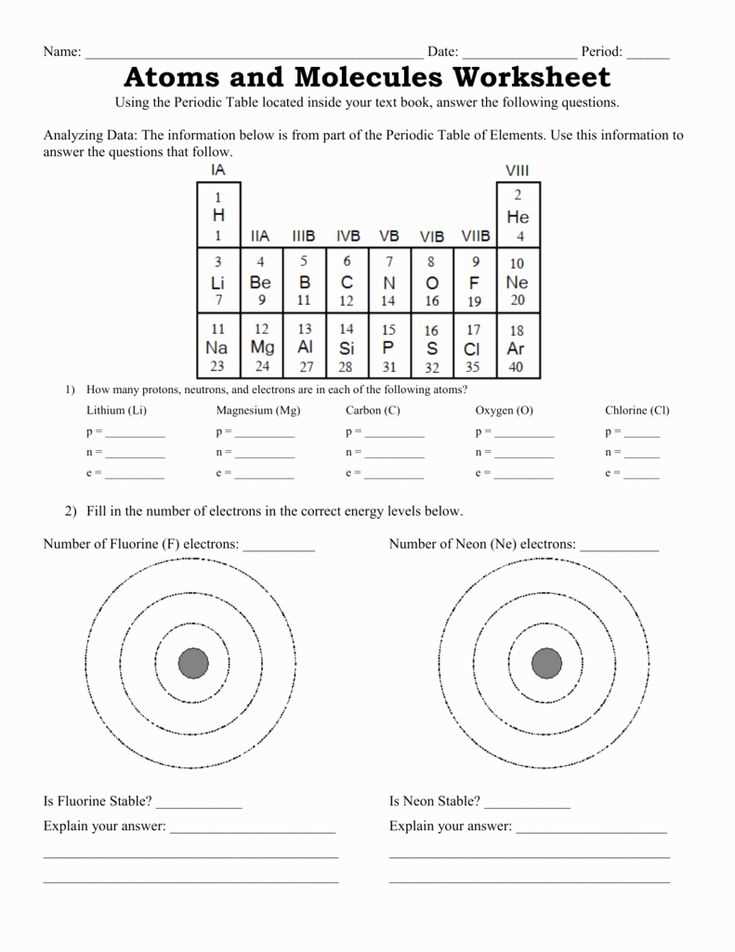
- Atomic number: The number of protons in an atom’s nucleus determines its atomic number and defines its element.
- Isotopes: Atoms of the same element can have different numbers of neutrons, resulting in different isotopes with slightly different atomic masses.
- Chemical reactions: Chemical reactions involve the rearrangement of atoms to form new substances, while preserving the total number and types of atoms.
The study of atoms and their properties has been crucial in various scientific fields, such as chemistry, physics, and materials science. It has allowed scientists to understand the behavior and properties of different substances, as well as develop technologies and applications that have transformed our daily lives.
What is an atom?
An atom is the smallest unit of a chemical element that retains the chemical properties of that element. It is composed of subatomic particles, including protons, neutrons, and electrons. These particles are bound together by the electromagnetic force, which holds the nucleus (composed of protons and neutrons) at the center of the atom, while the electrons orbit around it.
Protons are positively charged particles found in the nucleus of an atom. They carry a fundamental positive electric charge and have a mass approximately equal to that of a neutron.
Neutrons, on the other hand, are electrically neutral particles found in the nucleus of an atom. They do not have a charge and have a mass approximately equal to that of a proton.
Electrons are negatively charged particles that orbit around the nucleus in different energy levels or shells. They are much smaller and lighter than protons and neutrons, and they determine the chemical behavior of an atom through their interactions with other atoms.
Anatomical structure of an atom:
- Nucleus: The central part of an atom, composed of protons and neutrons.
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles found in the nucleus.
- Electrons: Negatively charged particles that orbit around the nucleus.
The study of atoms and their behavior is crucial to understanding the fundamental principles of chemistry and physics. By understanding the structure and properties of atoms, scientists have been able to develop a wide range of applications, from medicine and materials science to energy production and technology.
Early theories about atoms
The concept of atoms has been around for thousands of years, with early theories about atoms being developed by several ancient civilizations.
Greek philosophers: One of the earliest recorded theories about atoms comes from the ancient Greeks. The philosopher Democritus proposed that everything in the universe is made up of tiny, indivisible particles called atoms. He believed that atoms were constantly moving and could not be destroyed. However, this theory was not widely accepted at the time, as it went against the prevailing belief in the four classical elements of earth, air, fire, and water.
Indian philosophers: In ancient India, the concept of atoms was also explored. The philosopher Kanada, who lived around 6th century BCE, developed the atomic theory known as Vaisheshika. He believed that atoms were the smallest possible units of matter and that they combined to form different substances. Kanada’s atomic theory was further developed by other Indian philosophers, including Acharya Goutam and Acharya Nagarjuna.
Despite these early theories, it wasn’t until the 19th century that modern atomic theory began to take shape. The discoveries of John Dalton, J.J. Thomson, and Ernest Rutherford laid the foundation for our current understanding of atoms and their structure. Today, atoms are seen as the building blocks of matter and are essential to our understanding of chemistry and physics.
Ancient History of Atoms
The concept of atoms has been around for thousands of years, dating back to ancient civilizations such as the Greeks and Indians. These early thinkers speculated about the nature of matter and put forth various ideas about the fundamental building blocks of the universe. One of the key figures in ancient atomism was Democritus, a Greek philosopher who proposed that all matter is composed of tiny, indivisible particles called atoms.
Democritus believed that atoms were indestructible and eternal, and that they differed in shape and size. He imagined that these atoms were constantly in motion, and that their interactions with each other determined the properties of different types of matter. However, Democritus did not have any experimental evidence to support his ideas, so they remained purely philosophical.
It was not until much later, in the 18th and 19th centuries, that scientists began to develop a more concrete understanding of atoms. This period, known as the Scientific Revolution, saw the emergence of new experimental techniques and the formulation of various atomic theories.
John Dalton, an English chemist, is often credited with laying the foundation for modern atomic theory. In the early 19th century, Dalton proposed that atoms are indivisible and that different elements are composed of atoms with different masses. His theory also stated that atoms combine in fixed ratios to form compounds, and that chemical reactions involve the rearrangement of atoms.
Since Dalton’s time, our understanding of atoms has continued to evolve through the work of scientists such as J.J. Thomson, Ernest Rutherford, and Niels Bohr. These researchers made groundbreaking discoveries about the structure and behavior of atoms, leading to the development of quantum mechanics and the concept of the atomic nucleus.
- J.J. Thomson discovered the electron, a negatively charged particle that orbits the nucleus of an atom.
- Ernest Rutherford conducted the famous gold foil experiment, which revealed that atoms have a small, dense nucleus at their center.
- Niels Bohr proposed a new model of the atom, in which electrons occupy specific energy levels or shells around the nucleus.
Today, with the help of advanced technologies such as electron microscopes and particle accelerators, scientists have been able to study atoms in even greater detail. Our understanding of atomic structure and behavior continues to expand, as we explore the realms of subatomic particles and atomic interactions.
Ancient Greek philosophers and their ideas about atoms
The ancient Greek philosophers were some of the first to propose ideas about the nature of atoms. These philosophers, including Democritus and Leucippus, believed that everything in the universe was made up of tiny, indivisible particles they called “atoms.” They believed that atoms were eternal and indestructible, and that they combined and separated in different ways to form all matter. This idea of atoms as the building blocks of the physical world was revolutionary at the time and laid the groundwork for modern atomic theory.
Democritus was one of the most influential ancient Greek philosophers when it came to the idea of atoms. He believed that atoms were constantly moving in an empty void, colliding and combining to form matter. According to Democritus, these atoms were varied in shape and size, and their different combinations and arrangements gave rise to the different properties and characteristics of matter. He also believed that the soul itself was made up of atoms, a concept that was quite radical for his time.
Leucippus, another ancient Greek philosopher, expanded upon the ideas of Democritus. He believed that atoms were infinite in number and were in constant motion. Leucippus also proposed the concept of the void, a space in which atoms could move freely without obstruction. He believed that the void was necessary for the motion and interaction of atoms, and that without it, the universe would be static and motionless.
Despite their limited scientific tools and knowledge, these ancient Greek philosophers made significant contributions to our understanding of atoms and the nature of matter. Their ideas laid the foundation for the development of atomic theory and set the stage for future scientific discoveries in the field of chemistry and physics.
Indian and Chinese contributions to the concept of atoms
The concept of atoms has been prevalent in Indian and Chinese philosophy and scientific thought for centuries. Both ancient civilizations made significant contributions to our understanding of atoms, although their approaches and theories differed.
In India, the concept of atoms, or “anu” as it is known in Sanskrit, can be traced back to around 600 BCE. The Indian philosopher Kanada proposed that all matter is composed of eternal and indivisible particles called anu. These anu were believed to have different properties and could combine to form different substances. Kanada’s atomic theory relied on the idea that the universe is composed of an infinite number of anu, which are constantly in motion.
- Kanada: A key figure in the development of Indian atomic theory, proposed that all matter is made up of anu, or atoms, which are eternal and indivisible particles.
- Acharya Kanad: Another important philosopher in India, suggested that atoms have different properties and can combine to form various substances.
In China, the concept of atoms was explored in relation to the philosophical concept of Qi, which refers to the fundamental energy that flows through all things. Chinese philosophers and alchemists believed that matter is made up of tiny particles called qi, which are constantly vibrating and interacting with each other. These qi particles were similar to the idea of atoms, as they were considered to be indivisible and fundamental building blocks of matter.
- Qi: Chinese philosophers and alchemists developed the concept of qi, which can be seen as analogous to atoms. Qi particles were believed to be the fundamental building blocks of matter.
- Chinese alchemy: Chinese alchemists explored the idea of qi particles and their interactions, contributing to the development of the concept of atoms.
While Indian and Chinese contributions to the concept of atoms may have been rooted in philosophy and metaphysics, they laid the foundation for future scientific theories and discoveries. The ideas put forth by ancient Indian and Chinese thinkers continue to influence our understanding of matter and the atomic world.
Dalton’s atomic theory

Dalton’s atomic theory, proposed by John Dalton in the early 19th century, revolutionized the way scientists understood the nature of matter. Dalton’s theory provided a foundational framework for the field of chemistry and laid the groundwork for the development of modern atomic theory.
Key concepts of Dalton’s atomic theory:
- Atoms are indivisible and indestructible: According to Dalton, all matter is made up of tiny, indivisible particles called atoms. These atoms are indestructible and cannot be broken down into smaller pieces.
- Atoms of the same element are identical: Dalton proposed that all atoms of a given element are identical in their properties, such as mass and chemical behavior. Atoms of different elements, on the other hand, have different properties.
- Atoms combine in whole number ratios: Dalton suggested that atoms combine with each other to form compounds in simple, whole number ratios. For example, if compound A is formed by the combination of atoms X and Y, then the ratio of the number of atoms of X to the number of atoms of Y in compound A will always be in simple, whole numbers.
- Chemical reactions involve rearrangement of atoms: According to Dalton, chemical reactions occur when atoms rearrange themselves to form new compounds. During a chemical reaction, atoms are neither created nor destroyed; they simply rearrange to form different combinations.
Overall, Dalton’s atomic theory provided a comprehensive explanation of the behavior and structure of matter at the atomic level. Although some aspects of Dalton’s theory have been updated and refined with advancements in scientific knowledge, his contributions remain fundamental to our understanding of atomic structure and chemical reactions.