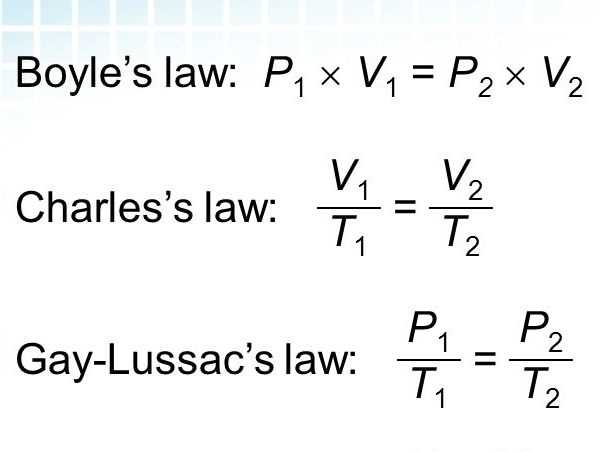
Understanding the principles behind Boyle’s Law and Charles’ Law is essential for any student studying physics or chemistry. These two gas laws explore the relationships between pressure, volume, and temperature, and provide a foundation for understanding the behavior of gases.
Boyle’s Law, named after the scientist Robert Boyle, states that the pressure of a gas is inversely proportional to its volume, when temperature is held constant. This law can be represented by the equation P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Charles’ Law, which is named after the French scientist Jacques Charles, states that the volume of a gas is directly proportional to its absolute temperature, when pressure is held constant. This can be represented by the equation V1 / T1 = V2 / T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
This answer key for the Student Exploration: Boyle’s Law and Charles’ Law Gizmo provides students with the opportunity to practice applying these principles and solving problems based on the laws. It offers step-by-step explanations and solutions for each question, allowing students to check their understanding and learn from any mistakes they may have made.
Student Exploration: Boyle’s Law and Charles Law Answer Key

Boyle’s Law and Charles Law are two important concepts in the field of gas laws and are often studied in chemistry classes. Boyle’s Law states that the pressure of a gas is inversely proportional to its volume, when the temperature and amount of gas are held constant. This can be expressed mathematically as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume. Charles Law, on the other hand, states that the volume of a gas is directly proportional to its temperature, when the pressure and amount of gas are held constant. Mathematically, it can be represented as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
In the Student Exploration: Boyle’s Law and Charles Law activity, students were given the opportunity to explore these laws through virtual experiments. They used an online simulation to vary the pressure, volume, and temperature of a gas and observe the resulting changes. They also measured the pressure and volume of a gas at different temperatures and used the data to calculate the value of absolute zero, the temperature at which the volume of a gas theoretically becomes zero.
Answer Key:
- Question 1: According to Boyle’s Law, how does the pressure of a gas change when its volume decreases? The pressure of a gas increases when its volume decreases.
- Question 2: According to Boyle’s Law, how does the pressure of a gas change when its volume increases? The pressure of a gas decreases when its volume increases.
- Question 3: According to Charles Law, how does the volume of a gas change when its temperature increases? The volume of a gas increases when its temperature increases.
- Question 4: According to Charles Law, how does the volume of a gas change when its temperature decreases? The volume of a gas decreases when its temperature decreases.
- Question 5: What is the relationship between the pressure and volume of a gas, according to Boyle’s Law? The pressure and volume of a gas are inversely proportional to each other.
By conducting virtual experiments and analyzing the data, students were able to understand and apply the principles of Boyle’s Law and Charles Law. This activity provided them with a hands-on experience in learning these fundamental concepts in gas laws, which are crucial in various scientific and industrial applications.
Understanding Boyle’s Law
Boyle’s Law is a fundamental principle in the field of physics and chemistry that describes the relationship between the pressure and volume of a gas at constant temperature. It states that the pressure of a gas is inversely proportional to its volume when temperature is held constant. In other words, as the volume of a gas decreases, its pressure increases, and vice versa.
According to Boyle’s Law, if the volume of a gas is halved, its pressure will double, and if the volume is doubled, the pressure will be halved. This relationship can be mathematically represented using the equation P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Key Concepts:
- In a closed system, the total amount of gas remains constant.
- Temperature must be kept constant for Boyle’s Law to apply.
- The gas must behave ideally, meaning it follows the assumptions of an ideal gas.
- Boyle’s Law can be used to predict the behavior of gases in various situations, such as changes in pressure and volume.
Understanding Boyle’s Law is essential for many practical applications in fields such as engineering, medicine, and environmental science. For example, it helps engineers design efficient pneumatic systems, allows medical professionals to monitor the respiratory function of patients, and assists scientists in studying the behavior of gases in different environmental conditions.
Overall, Boyle’s Law provides a fundamental understanding of how the pressure and volume of a gas are related. By applying this knowledge, scientists and engineers can make accurate predictions and develop innovative solutions to various real-world problems.
Exploring Charles Law
In the study of gases, Charles’ Law, also known as the Law of Volumes, describes the relationship between the volume and temperature of a gas when pressure and amount of gas are held constant. According to Charles’ Law, as the temperature of a gas increases, the volume of the gas also increases, and vice versa.
Charles’ Law equation: V1/T1 = V2/T2
This equation can be used to calculate the final volume (V2) of a gas at a given temperature (T2), if the initial volume (V1) and temperature (T1) are known. It can also be used to compare the effect of temperature changes on the volume of a gas by comparing the ratio of the initial and final temperatures to the ratio of the initial and final volumes.
To demonstrate Charles’ Law, an experiment can be conducted using a fixed amount of gas (such as air) in a container with a movable piston. The temperature of the gas can be changed while keeping the pressure constant, and the corresponding changes in volume can be observed. The results of the experiment should show that as the temperature increases, the volume of the gas expands, and as the temperature decreases, the volume of the gas contracts.
- Practical applications:
- – Charles’ Law is important in various fields, such as in the design and operation of engines, refrigeration systems, and weather balloons.
- – It is also used in the study of gas behavior and thermodynamics.
- – Understanding Charles’ Law can help in predicting the behavior of gases in different conditions and designing systems that rely on gas volume changes.
Experimental Procedure for Boyle’s Law

Boyle’s law is a fundamental principle in gas behavior that states the pressure of a gas is inversely proportional to its volume when temperature and the number of gas particles are held constant. In this experiment, we will investigate Boyle’s law by measuring the relationship between the pressure and volume of a gas.
Materials:
- Gas syringe
- Pressure gauge
- Gas sample
- Stopwatch
Procedure:
- Set up the apparatus by connecting the gas syringe to the pressure gauge.
- Ensure that the gas syringe is empty and the pressure gauge is calibrated to zero.
- Apply a constant pressure to the gas sample by squeezing the syringe.
- Measure and record the initial volume of the gas sample.
- Release the pressure slowly by allowing the syringe to expand.
- Measure and record the final volume of the gas sample.
- Repeat steps 3-6 for different pressure values.
Results:
Record the pressure and volume values for each trial in a table. Calculate the inverse of the volume for each pressure value. Plot a graph of pressure versus the inverse of volume. The graph should show a linear relationship, indicating Boyle’s law is valid.
Conclusion:
Based on the experiment’s results, we can conclude that there is an inverse relationship between the pressure and volume of a gas when temperature and the number of gas particles are kept constant, in accordance with Boyle’s law. The graph of pressure versus the inverse of volume shows a linear trend, confirming the validity of Boyle’s law.
Answer Key for Boyle’s Law Worksheet
In the Boyle’s Law Worksheet, students are required to solve problems related to Boyle’s Law, which states that the pressure and volume of a gas are inversely proportional when the temperature and number of moles of gas remain constant. The worksheet provides several questions where students are asked to calculate the initial and final volume or pressure of a gas sample.
Here is the answer key for the Boyle’s Law Worksheet:
- Problem 1: The initial volume of a gas is 5 L with a pressure of 2 atm. According to Boyle’s Law, when the volume is doubled, the pressure is halved. Therefore, the final volume is 10 L and the final pressure is 1 atm.
- Problem 2: The initial pressure of a gas is 3 atm with a volume of 8 L. When the pressure is tripled, the volume is reduced to one-third. Therefore, the final pressure is 9 atm and the final volume is 2.67 L.
- Problem 3: The initial volume of a gas is 10 L with a pressure of 4 atm. According to Boyle’s Law, when the volume is halved, the pressure is doubled. Therefore, the final volume is 5 L and the final pressure is 8 atm.
- Problem 4: The initial pressure of a gas is 2 atm with a volume of 6 L. When the pressure is doubled, the volume is increased to twice its initial value. Therefore, the final pressure is 4 atm and the final volume is 12 L.
- Problem 5: The initial volume of a gas is 2 L with a pressure of 5 atm. According to Boyle’s Law, when the volume is tripled, the pressure is reduced to one-third. Therefore, the final volume is 6 L and the final pressure is 1.67 atm.
By solving these problems, students can practice applying Boyle’s Law and understand the relationship between pressure and volume in a gas sample. It is important for students to grasp this concept as it is fundamental to understanding the behavior of gases.
Experimental Procedure for Charles Law
In order to investigate Charles’ law, which states that the volume of a gas is directly proportional to its temperature, the following experimental procedure was followed:
Materials:
- Glass tube with a fixed volume
- Thermometer
- Bunsen burner
- Clamp
- Stopwatch
Procedure:
- Set up the Bunsen burner and light it.
- Attach the glass tube to the clamp and place it above the flame.
- Wait for the gas inside the glass tube to reach a constant volume.
- Using the stopwatch, record the initial temperature of the gas.
- Keep the Bunsen burner lit and start heating the gas.
- Continue heating the gas for a fixed amount of time, such as 2 minutes.
- After the fixed amount of time, record the final temperature of the gas.
- Calculate the change in temperature by subtracting the initial temperature from the final temperature.
- Measure the change in volume of the gas by subtracting the initial volume from the final volume.
- Plot a graph of the change in volume versus the change in temperature.
Results:
The results of the experiment can be analyzed by examining the graph of the change in volume versus the change in temperature. If the volume and temperature are indeed directly proportional, the graph should be a straight line. Any deviation from a straight line would suggest a violation of Charles’ law. By analyzing the slope of the graph, the proportionality constant can be determined, which relates the change in volume to the change in temperature.
Answer Key for Charles Law Worksheet

1. Charles’s law states that the volume of a gas is directly proportional to its temperature, assuming constant pressure and amount of gas.
In the given worksheet, students were asked to solve problems related to Charles’s law. The first question asked them to calculate the new volume of a gas if its initial volume was 5 liters and its temperature increased from 20°C to 40°C. Using Charles’s law equation, V₁/T₁ = V₂/T₂, the students had to plug in the given values and solve for V₂. The correct answer would be the new volume of the gas at 40°C.
2. The second question asked students to determine the temperature at which a gas would have a volume of 10 liters, given that its initial volume was 5 liters and its temperature was 20°C.
To solve this problem, students needed to rearrange the Charles’s law equation and solve for T₂. The equation can be rewritten as T₂ = T₁(V₂/V₁), where T₁ is the initial temperature, V₁ is the initial volume, V₂ is the desired volume, and T₂ is the unknown temperature. By plugging in the appropriate values, students could find the temperature at which the gas would have a volume of 10 liters.
3. The third question involved determining the final volume of a gas if its initial volume was 2 liters, its temperature was 30°C, and its temperature increased to 40°C.
Using Charles’s law equation, V₁/T₁ = V₂/T₂, the students needed to find V₂, the final volume of the gas at 40°C. They could do this by plugging in the appropriate values and solving for V₂. The correct answer would be the final volume of the gas at 40°C.
By correctly solving these problems, students would demonstrate their understanding of Charles’s law and its application in calculating changes in volume due to temperature variations. These calculations are essential in understanding the behavior of gases and can be used to predict how changes in temperature will affect the volume of a gas.