
Stoichiometry is a fundamental concept in chemistry that deals with the numerical relationships between reactants and products in a chemical reaction. Understanding stoichiometry is essential for students to analyze and solve problems in chemistry. Chapter 12 of your chemistry textbook provides an in-depth exploration of stoichiometry, and taking a test on this chapter can be a challenge. However, with the right preparation and a comprehensive answer key, you can be confident in your understanding of the subject matter and excel on the test.
This article will provide you with a comprehensive answer key for the Chapter 12 stoichiometry test. It will cover key concepts such as molar ratios, mole-to-mole calculations, limiting reagents, and percent yield. Each question will be explained in detail, providing step-by-step solutions and explanations.
By studying the answer key and understanding the reasoning behind each solution, you will not only be able to perform well on the test but also gain a deeper grasp of stoichiometry. With this knowledge, you will be able to approach future stoichiometry problems with confidence and apply your understanding to real-world scenarios. So, dive into the Chapter 12 stoichiometry test answer key and unlock the secrets to mastering this fundamental concept in chemistry!
Chapter 12 Stoichiometry Test Answer Key

In Chapter 12, we explored stoichiometry, which is the study of the quantitative relationships between substances in chemical reactions. This test allows us to assess your understanding of stoichiometry concepts and calculations. Below is the answer key to help you check your answers and evaluate your performance on the test.
1. Convert the following mass of carbon dioxide, in grams, to moles:
Given mass of carbon dioxide: 44.01 g
Answer: 1 mole of carbon dioxide
2. Calculate the number of moles of oxygen gas required to react with 3 moles of hydrogen gas in the following reaction:
2H2 + O2 → 2H2O
Given moles of hydrogen gas: 3 moles
Answer: 1.5 moles of oxygen gas
3. Determine the limiting reagent in the following reaction:
2Al + 3Cl2 → 2AlCl3
Given grams of aluminum: 50 g
Given grams of chlorine: 100 g
Answer: Aluminum is the limiting reagent
4. Calculate the theoretical yield of calcium carbonate, in grams, when 10 moles of calcium oxide react with excess carbon dioxide in the following reaction:
CaO + CO2 → CaCO3
Given moles of calcium oxide: 10 moles
Answer: 330 g of calcium carbonate
5. Determine the percent yield of a reaction if the actual yield is 25 g and the theoretical yield is 30 g:
Given actual yield: 25 g
Given theoretical yield: 30 g
Answer: 83.33% yield
These are just a few examples of the questions and answers that may appear in a stoichiometry test. It is important to practice these calculations to solidify your understanding of the subject. Keep studying and good luck with your future stoichiometry endeavors!
Understanding Stoichiometry
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows us to determine the amount of each substance involved in a reaction, as well as predict the outcome of the reaction.
At its core, stoichiometry is based on the principle of mass conservation. According to the law of conservation of mass, matter is neither created nor destroyed in a chemical reaction. This means that the total mass of the reactants must be equal to the total mass of the products.
In order to perform stoichiometric calculations, we need to know the balanced chemical equation for the reaction. A balanced equation shows the relative number of molecules or formula units of each substance involved in the reaction. It is important to note that a balanced equation is based on the law of definite proportions, which states that a chemical compound always contains the same elements in the same proportions by mass.
Stoichiometric calculations involve converting between mass, moles, and number of particles. The basic steps in solving stoichiometry problems include: identifying the known and unknown quantities, determining the molar ratios between the substances in the balanced equation, calculating the moles of the known substance, using the mole ratio to find the moles of the unknown substance, and finally converting the moles of the unknown substance to the desired units (mass, volume, etc.).
Understanding stoichiometry is crucial in many branches of chemistry, including analytical chemistry, environmental chemistry, and materials science. It allows us to make informed decisions regarding the amounts of reactants to use, the efficiency of a reaction, and the yield of a product. By applying stoichiometry, chemists can optimize chemical processes and minimize waste.
In conclusion, stoichiometry is a powerful tool that enables chemists to quantitatively analyze chemical reactions. It provides a framework for understanding the relationship between reactants and products, and allows for the prediction and calculation of the quantities involved. Through stoichiometric calculations, chemists can gain insights into the fundamental principles of chemistry and make practical applications in various fields.
The Importance of Balancing Equations
The process of balancing chemical equations is essential in chemistry because it allows us to interpret and predict the outcome of chemical reactions. Balancing equations involves adjusting the coefficients of the reactants and products to ensure that the law of conservation of mass is followed. This law states that matter cannot be created or destroyed in a chemical reaction, only rearranged.
By balancing equations, we can determine the stoichiometry, or the quantitative relationships between reactants and products. This information is crucial in understanding the amount of each substance involved in a reaction, as well as the ratio in which they are consumed and produced. Without balanced equations, it would be impossible to accurately calculate the amount of reactants needed or the amount of products obtained.
Furthermore, balanced equations provide a basis for calculating the theoretical yield of a reaction. The theoretical yield is the maximum amount of product that can be obtained based on the stoichiometry of the reaction. By knowing this value, chemists can evaluate the efficiency of a reaction and make adjustments to improve its yield.
In addition to their practical applications, balancing equations also helps us gain a deeper understanding of the underlying principles of chemistry. It allows us to visualize the transfer of atoms and understand how different elements interact and combine to form new substances. Balancing equations is a fundamental skill that forms the basis of many more advanced topics in chemistry, such as reaction kinetics and thermodynamics.
- Key phrases: balancing chemical equations, law of conservation of mass, stoichiometry, quantitative relationships, theoretical yield, efficiency of a reaction, transfer of atoms, reaction kinetics, thermodynamics.
Determining Stoichiometric Ratios
In chemical reactions, stoichiometry plays a crucial role in determining the quantities of reactants and products involved. Stoichiometric ratios provide a way to determine the exact amounts of substances needed or produced in a reaction.
The stoichiometric ratio is obtained from the balanced chemical equation, which shows the relationship between the reactants and products. It represents the mole-to-mole ratio between the different substances involved in the reaction.
To determine the stoichiometric ratio, one needs to examine the coefficients of the reactants and products in the balanced equation. The coefficient represents the number of moles of each substance involved in the reaction. By comparing the coefficients of the reactants and products, one can calculate the stoichiometric ratio.
The stoichiometric ratio is used in stoichiometry calculations, such as determining the limiting reactant, calculating theoretical yield, and finding the percent yield. It allows chemists to accurately predict how much of each substance is needed or produced in a reaction, which is crucial for practical applications.
Overall, determining stoichiometric ratios is an essential step in understanding and predicting the quantities of substances involved in chemical reactions. It enables chemists to perform calculations and make accurate predictions about the outcome of a reaction, making it a fundamental concept in stoichiometry.
Calculating the Amount of Reactant or Product
When studying stoichiometry, one of the important calculations involves determining the amount of reactant or product involved in a chemical reaction. This calculation is based on the stoichiometric coefficients, which represent the ratios of moles of substances in a balanced chemical equation.
To calculate the amount of reactant consumed or product formed, you need to use the given information, such as the mass of a reactant or the volume of a solution, and convert it to moles using the molar mass or the molarity of the substance. Then, using the stoichiometric coefficients, you can determine the ratio between the substance of interest and the other substances involved in the reaction.
Example: Let’s consider the following balanced equation: 2H2 + O2 → 2H2O. If we have 4 moles of H2 and we want to determine the amount of water (H2O) produced, we can use the stoichiometric coefficients to calculate it. Since the ratio between H2 and H2O is 2:2, we can conclude that for every 2 moles of H2, 2 moles of H2O are formed. Therefore, if we have 4 moles of H2, we will have 4 moles of H2O.
To summarize, calculating the amount of reactant or product in stoichiometry involves converting the given information to moles, using the stoichiometric coefficients to determine the ratio, and then applying that ratio to calculate the desired amount. It is important to remember the importance of balanced equations and the stoichiometry coefficients in these calculations.
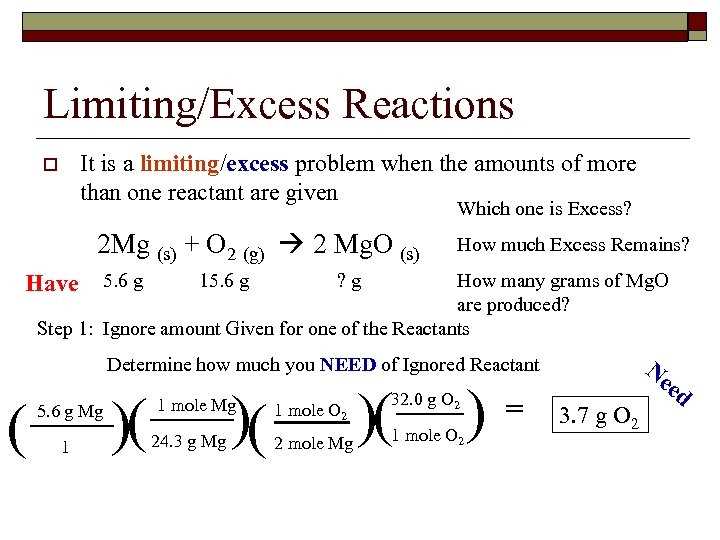
Limiting Reactants and Excess Reactants
In chemical reactions, the reactants are the substances that undergo a chemical change to produce products. However, not all reactants are consumed in equal amounts. The reactant that is completely consumed is called the limiting reactant, while the reactant that is present in excess is called the excess reactant.
The limiting reactant is the key factor that determines the maximum amount of product that can be formed in a reaction. It is determined by calculating the moles of each reactant and comparing them to the stoichiometric ratio in the balanced chemical equation. The reactant that produces the smallest amount of product is the limiting reactant.
On the other hand, the excess reactant is the reactant that is left over after the reaction has gone to completion. It is not completely consumed and is present in excess quantities. The excess reactant is often used to calculate the amount of the limiting reactant that was actually consumed in the reaction.
To determine the amount of product formed in a reaction, it is crucial to identify the limiting reactant. This allows for accurate calculations of the amount of product that can be formed, as well as the amount of excess reactant that is left over.
In summary, the limiting reactant is the reactant that is completely consumed and determines the maximum amount of product that can be formed. The excess reactant is the reactant that is present in excess and is not completely consumed. Calculating the limiting reactant and excess reactant is essential for accurate stoichiometric calculations in chemical reactions.
Finding the Percent Yield
When conducting chemical reactions, it is important to measure the efficiency of the reaction. One way to do this is by calculating the percent yield. Percent yield is a measure of how much product is actually produced compared to the theoretical yield, which is the maximum amount of product that can be obtained based on the stoichiometry of the reaction.
To calculate the percent yield, you need to know the actual yield, which is the amount of product that is actually obtained experimentally. This can be determined through experimental techniques such as weighing the product or measuring the volume of gas produced. The actual yield is then divided by the theoretical yield and multiplied by 100 to get the percent yield.
Percent Yield = (Actual Yield / Theoretical Yield) * 100%
It is important to note that the percent yield is never 100% in practice. There are several factors that can contribute to a lower yield, such as incomplete reactions, side reactions, and loss of product during purification or filtration. These factors can result in a percent yield that is less than 100%.
In order to improve the percent yield, various techniques can be employed, such as optimizing reaction conditions, using catalysts, and improving purification techniques. By maximizing the efficiency of the reaction, a higher percent yield can be achieved.
Overall, calculating the percent yield is an important aspect of stoichiometry as it allows chemists to evaluate the efficiency of a reaction and make improvements to optimize the yield of the desired product.
Solving Stoichiometry Problems
In chemistry, stoichiometry is the branch that deals with the quantitative relationship between reactants and products in a chemical reaction. It allows us to predict the amount of products produced or reactants needed in a given reaction. Solving stoichiometry problems involves using balanced chemical equations and performing calculations based on the stoichiometric ratios.
To solve stoichiometry problems, the first step is to write a balanced chemical equation for the reaction. This equation shows the mole ratios between reactants and products. The coefficients in the balanced equation represent the mole ratios, which can be used to convert between moles of different substances involved in the reaction.
Once the balanced chemical equation is established, the next step is to determine the given information. This includes the amount of one substance (given in moles, grams, or other units) and the target substance to be calculated. It is important to convert all given information into moles for accurate calculations.
Using the stoichiometric ratios from the balanced equation, the next step is to set up a stoichiometry calculation. This involves using dimensional analysis to convert from the given substance to the target substance. The stoichiometric ratios serve as conversion factors in these calculations, allowing us to determine the amount of the target substance.
Finally, the last step is to perform the calculations and obtain the final answer. It is important to keep track of units and significant figures throughout the calculations. The final answer should be rounded to the appropriate number of significant figures based on the least precise measurement in the problem.
Solving stoichiometry problems requires a solid understanding of balanced chemical equations and the ability to use stoichiometric ratios to convert between different substances. Practice and familiarity with the process is key to becoming proficient in solving stoichiometry problems.