Unit testing is an important aspect of any scientific experiment. It helps validate the laws and principles that govern various phenomena, such as gas behavior. Gas laws play a crucial role in understanding the physical properties of gases, their interrelationships, and their behavior under different conditions.
In this article, we will delve into the process of unit testing gas laws and its significance in experimental design and data analysis. We will explore the different gas laws, including Boyle’s Law, Charles’ Law, and the Ideal Gas Law, and discuss the importance of unit testing in ensuring accurate measurements and reliable results.
Unit testing gas laws involves conducting experiments that involve the manipulation of variables such as pressure, volume, and temperature. By systematically varying these parameters and observing the corresponding changes, scientists can verify the accuracy and validity of the gas laws. This process helps uncover any inconsistencies or discrepancies in the theoretical principles and provides valuable insights into the behavior of gases.
Furthermore, unit testing gas laws allows scientists to identify and understand the limitations of these laws under specific conditions. It helps verify the assumptions made in the derivation of these laws and provides a solid foundation for future research and experimentation. In addition, unit testing gas laws helps scientists develop more accurate models and theories that can better explain the behavior of gases, leading to advancements in various scientific disciplines.
Understanding the concept of unit testing gas laws
The gas laws, such as Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law, are fundamental principles in chemistry that describe the behavior of gases. Unit testing is an important tool in software engineering that helps ensure the correctness and reliability of code. By combining the concepts of unit testing and gas laws, we can verify the accuracy of calculations and predictions made by gas law formulas.
Unit testing gas laws involves creating test cases that validate the output of gas law calculations against expected results. For example, in Boyle’s Law, which states that the pressure of a gas is inversely proportional to its volume at constant temperature, we can create test cases with different sets of pressure and volume values. By calculating the expected result using the gas law formula and comparing it with the output of the tested code, we can determine if the implementation is correct.
Advantages of unit testing gas laws:
- Ensures the accuracy of gas law calculations
- Verifies the correctness of formula implementations
- Helps identify and fix bugs in the code
- Allows for the evaluation of edge cases and special scenarios
- Provides a means to validate the behavior of gas law simulations or calculators
By conducting unit tests on gas law calculations, we can have confidence in the accuracy of our code and the reliability of our results. It also allows us to have a deeper understanding of the underlying principles of gas laws and their mathematical formulations. Ultimately, unit testing gas laws helps ensure the quality of gas law-related software and promotes reliable scientific calculations in various fields, including chemistry, physics, and engineering.
Definition of unit testing
Unit testing is a software testing method in which individual units of source code are tested independently and isolated from the rest of the system. It focuses on the smallest testable parts of a program, typically functions or methods, to ensure that they work correctly and produce the expected output. In unit testing, each unit is tested in isolation by providing inputs and verifying the outputs, without considering the interactions with other units or the system as a whole.
Unit tests are written by the developers themselves and are typically automated to be executed repeatedly during the development process. They are designed to be specific, simple, and easy to understand, with clear inputs and expected outputs. By testing each unit separately, any bugs or issues can be identified and fixed early in the development cycle, preventing potential problems in the overall system.
Unit testing is an essential practice in software development as it helps ensure the quality and reliability of the code. It aids in identifying and resolving issues early, which can save time and effort in the long run. Additionally, unit tests provide a safety net for developers when making changes or refactoring code, as they can quickly verify that the existing functionality still works as expected.
The process of unit testing involves writing test cases, executing them, and analyzing the results. Test cases are written to cover different scenarios and edge cases, ensuring that the unit handles all possible inputs correctly. Automated testing frameworks and tools are commonly used to streamline the process and provide reporting and analysis capabilities.
Overall, unit testing plays a crucial role in ensuring the correctness, stability, and maintainability of software systems. It helps developers identify and fix bugs early, promotes code quality, and provides confidence when making changes to the codebase.
Overview of gas laws
The study of gases is an important part of chemistry and physics. Gas laws provide a mathematical description of the behavior of gases under different conditions. These laws help scientists understand and predict how gases will react and behave in various situations.
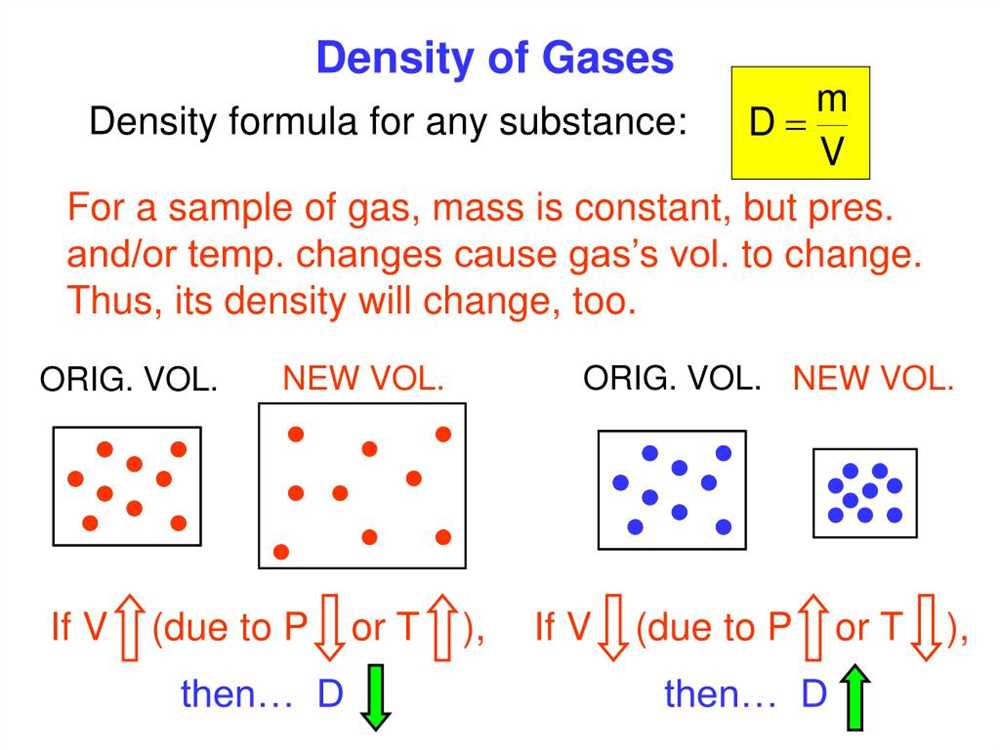
One of the most fundamental gas laws is Boyle’s law, which states that the pressure of a gas is inversely proportional to its volume, when temperature and amount of gas are constant. This law can be expressed mathematically as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
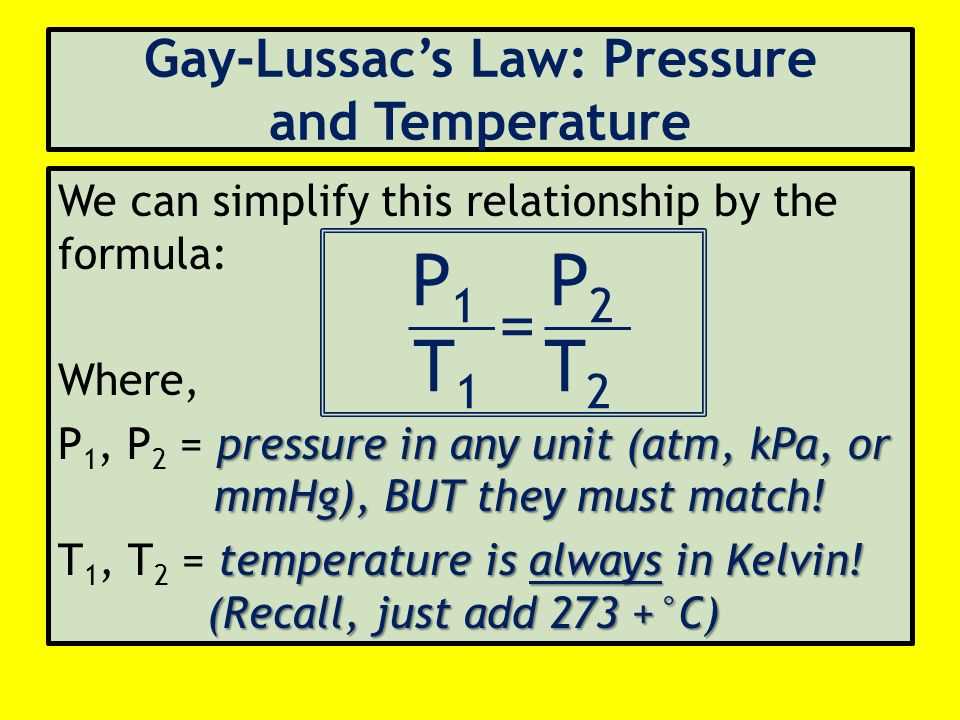
Another important gas law is Charles’ law, which describes the relationship between the volume and temperature of a gas, when pressure and amount of gas are constant. According to Charles’ law, the volume of a gas is directly proportional to its temperature. This law can be represented by the equation V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
Avogadro’s law states that equal volumes of gases at the same temperature and pressure contain the same number of particles (atoms, molecules, or ions). This law is based on the concept that gases are composed of individual particles that are in constant motion. Avogadro’s law can be expressed as V1/n1 = V2/n2, where V1 and n1 are the initial volume and number of particles, and V2 and n2 are the final volume and number of particles.
These gas laws, along with other principles such as Dalton’s law of partial pressures and the ideal gas law, help scientists understand the properties and behavior of gases in various scientific fields, such as chemistry, physics, and engineering. They are essential tools for calculating and predicting the behavior of gases in different experimental and real-life situations.
Importance of Unit Testing Gas Laws
Unit testing is an essential practice in the field of gas laws as it helps ensure the accuracy and reliability of the calculations and formulas used to describe the behavior of gases. Unit testing involves testing each individual component of the gas law equations and verifying that they produce the expected results. This process helps identify any errors or inconsistencies in the implementation of the gas laws, allowing for their correction and improvement.
The accurate measurement and prediction of gas properties are crucial in various scientific and industrial applications. Whether it is determining the pressure and volume relationships in a gas sample or calculating the amount of gas produced in a chemical reaction, the reliability of gas law calculations directly impacts the quality and validity of the results obtained. Unit testing allows for the validation of these calculations, ensuring that they conform to the established gas laws and yield precise and accurate results.
Additionally, unit testing in gas laws facilitates the identification and rectification of any coding errors or bugs in the software or programming used for these calculations. By systematically testing each component of the calculations, developers can detect any discrepancies between expected and actual outputs and trace them back to the specific lines of code responsible. This ensures the elimination of any errors or inconsistencies that may compromise the accuracy and reliability of the gas law calculations.
In conclusion, unit testing plays a vital role in the field of gas laws by ensuring the accuracy, reliability, and integrity of the calculations and formulas used. It helps identify and rectify any errors or inconsistencies in the implementation of the gas laws, ultimately leading to more precise and accurate results. By conducting thorough unit tests, scientists and engineers can increase their confidence in the gas law calculations, allowing for better decision-making and improved understanding of gaseous systems.
Ensuring accurate measurement and calculation
Accurate measurement and calculation are essential in unit testing gas laws to obtain reliable and precise results. It is crucial to ensure precise measurements of different variables, such as pressure, temperature, and volume, as any small error during measurement can lead to significant discrepancies in the final calculation.
One way to ensure accurate measurement is by using calibrated and standardized equipment. It is vital to regularly calibrate the instruments used in gas law experiments to minimize errors caused by equipment inaccuracies. Additionally, using instruments with high precision and resolution can help in obtaining more reliable measurements.
- Pressure: The pressure of the gas can be measured using instruments like a manometer or a pressure gauge. It is essential to zero the instrument before measurement to eliminate any systematic errors. Furthermore, ensuring a tight seal between the gas source and the instrument can prevent any leaks or pressure losses.
- Temperature: Precise temperature measurement is crucial in gas law calculations. Using a high-quality thermometer or a thermocouple that is properly calibrated can help in obtaining accurate temperature readings. It is also important to measure the temperature at the same location as the gas to avoid any temperature gradients.
- Volume: Accurate volume measurement can be achieved by using calibrated glassware, such as burettes or graduated cylinders. It is important to take into account any meniscus or parallax errors and ensure proper alignment while reading the volume.
Once the measurements are obtained, accurate calculations are necessary to apply the gas laws correctly. The correct equation and proper unit conversions should be used to carry out the calculations. It is essential to double-check the calculations to prevent any mistakes or rounding errors that may affect the final results.
In conclusion, ensuring accurate measurement and calculation is paramount in unit testing gas laws. By using calibrated equipment, taking precautions during measurement, and applying precise calculations, one can obtain reliable data and minimize errors in the experimental results. It is always necessary to maintain a high level of accuracy and precision to ensure the validity of the gas law experiments.
Identifying potential errors and inaccuracies
When conducting unit tests on gas laws, it is important to be aware of potential errors and inaccuracies that may arise. These errors can affect the reliability and accuracy of the results, and can be categorized into systematic and random errors.
Systematic errors
Systematic errors occur due to consistent biases in measurement or experimental setup. These errors can arise from equipment calibration issues, environmental factors, or procedural mistakes. For example, if the pressure gauge used in the experiment is not properly calibrated, it may consistently display incorrect readings, leading to inaccuracies in the results. To avoid systematic errors, it is essential to ensure the equipment is properly calibrated and that all experimental procedures are followed accurately.
Random errors
Random errors, on the other hand, are unpredictable and occur due to fluctuations or variations in measurement. These errors can arise from factors such as experimental uncertainties, limitations in measurement precision, or human errors. In gas law experiments, random errors can manifest as variations in temperature, pressure, or volume measurements. To minimize random errors, it is important to take multiple measurements and calculate the average, as well as to use precise instruments and techniques.
In addition to systematic and random errors, it is also crucial to consider any limitations inherent in the gas laws themselves. The ideal gas law, for example, assumes a number of idealized conditions that may not always hold true in real-life experiments. Factors such as non-ideal behavior of gases at high pressures or low temperatures can introduce inaccuracies in the results.
Overall, identifying and understanding potential errors and inaccuracies in unit tests on gas laws is crucial for obtaining reliable and accurate results. By being aware of systematic errors, random errors, and limitations in the gas laws, researchers can take appropriate measures to minimize errors and ensure the validity of their experimental findings.
Steps to conduct unit tests on gas laws
Unit tests on gas laws are crucial in order to validate the accuracy and reliability of the mathematical models used to describe the behavior of gases. These tests involve conducting experiments and comparing the observed results with the theoretical predictions based on the gas laws. Here are the steps to conduct unit tests on gas laws:
1. Define the objective of the test
The first step in conducting unit tests on gas laws is to clearly define the objective of the test. This involves determining which gas law or laws will be tested, and what specific aspect of the gas behavior will be investigated. For example, the objective may be to verify the relationship between pressure and volume described by Boyle’s law.
2. Design an experimental setup
Once the objective is defined, the next step is to design an experimental setup that allows for the measurement of relevant variables and the manipulation of gas conditions. This may involve using specialized apparatus such as pressure sensors, volume chambers, and temperature controllers to create controlled gas environments. The setup should be designed in a way that minimizes sources of error and provides accurate and reliable measurements.
3. Collect data
With the experimental setup in place, the next step is to collect data by conducting the experiments. This involves carefully following the procedures and systematically varying the parameters, such as pressure or volume, while keeping other variables constant. The measurements should be recorded accurately and with the appropriate precision to ensure reliable data collection.
4. Analyze the data
Once the data is collected, it needs to be analyzed to determine if it aligns with the theoretical predictions based on the gas laws being tested. This may involve performing mathematical calculations and plotting graphs to visualize the relationships between variables. Statistical analysis techniques can be used to assess the level of agreement between the observed data and the theoretical predictions.
5. Draw conclusions
Based on the analysis of the data, conclusions can be drawn regarding the validity of the gas laws being tested. If the observed data closely matches the theoretical predictions, it provides evidence that the gas laws accurately describe the behavior of the gases in the specific conditions tested. On the other hand, discrepancies between the observed and predicted data may indicate the need for further refinement of the gas law models or identification of other factors that affect gas behavior.
6. Report and document the results
Finally, the results of the unit tests should be reported and documented. This includes providing a detailed description of the experimental setup, the collected data, the analysis performed, and the conclusions drawn. It is important to accurately record all the information to ensure transparency, reproducibility, and facilitate future reference for further research or validation studies.