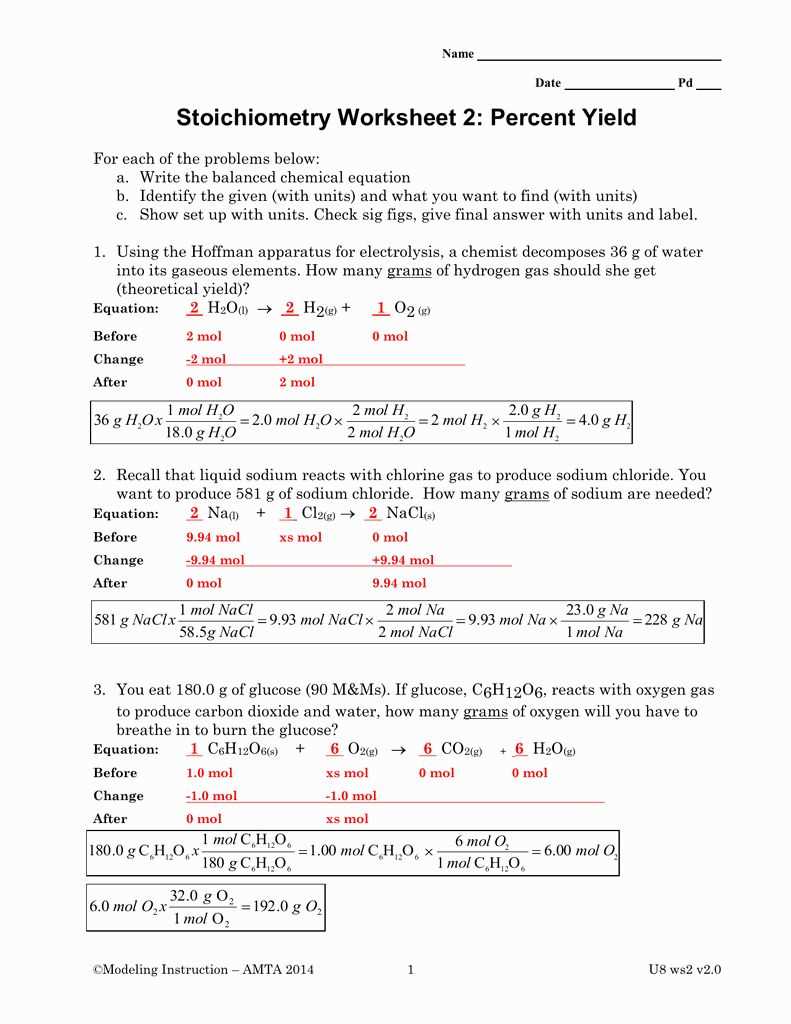
Gas stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in gas phase reactions. It involves calculating the amounts of reactants consumed and products formed using stoichiometric ratios.
A gas stoichiometry worksheet is a useful tool for practicing these calculations. It typically contains a series of problems that require the application of stoichiometry principles to solve. These problems often involve balancing chemical equations, determining the limiting reactant, and calculating the amounts of reactants and products.
An answer key is an essential component of a gas stoichiometry worksheet. It provides the correct solutions and explanations for each problem, allowing students to check their work and learn from any mistakes. The answer key also serves as a valuable study resource, providing examples of how to approach and solve different types of gas stoichiometry problems.
By using the gas stoichiometry worksheet answer key, students can gain a better understanding of the principles and calculations involved in gas stoichiometry. This knowledge is crucial for success in chemistry and other related fields, as it forms the basis for determining reaction yields and understanding the behavior of gases in various chemical processes.
Gas Stoichiometry Worksheet Answer Key
In gas stoichiometry, we use the principles of stoichiometry to calculate the amounts of reactants and products in a chemical reaction involving gases. This involves using the balanced chemical equation, the ideal gas law, and other gas laws to determine the quantities of gases involved in the reaction.
The gas stoichiometry worksheet provides a series of problems that require calculations using these principles. The answer key to the worksheet includes the solutions to these problems, allowing students to check their work and practice their understanding of gas stoichiometry.
Example Problem:
1. Consider the following balanced chemical equation:
2 H2(g) + O2(g) → 2 H2O(g)
If 4.50 moles of hydrogen gas (H2) react with excess oxygen gas (O2), how many moles of water (H2O) are produced?
Answer:
Using the balanced chemical equation, we can see that the stoichiometric ratio between H2 and H2O is 2:2. This means that for every 2 moles of H2, 2 moles of H2O are produced.
Therefore, if we have 4.50 moles of H2, we can calculate the moles of H2O using the following proportion:
(4.50 moles H2) * (2 moles H2O / 2 moles H2) = 4.50 moles H2O
Therefore, 4.50 moles of water (H2O) are produced.
The gas stoichiometry worksheet answer key provides the solutions to these types of problems, allowing students to practice their skills and check their understanding of gas stoichiometry calculations.
What is Gas Stoichiometry?
Gas stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in gas-phase chemical reactions. It involves using the principles of stoichiometry, which is the study of the quantitative relationships between substances in a chemical reaction, to analyze and predict the amounts of gases involved.
In gas stoichiometry, the molar ratios between reactants and products are determined based on the balanced chemical equation for the reaction. These molar ratios can then be used to calculate the amounts of gases involved, such as the volume, mass, or moles of a gas in a reaction.
Gas stoichiometry involves the following key concepts:
- Mole-to-mole ratios: These ratios are determined based on the stoichiometry of the reaction and are used to convert between the moles of different gases involved in the reaction.
- Gas laws: Gas stoichiometry relies on various gas laws, such as Boyle’s law, Charles’s law, Avogadro’s law, and the ideal gas law, to relate the volume, pressure, temperature, and moles of gases.
- Gas density: Gas stoichiometry also involves the calculation of gas density, which is the mass of a gas per unit volume, and is used to determine the molar mass of a gas.
- Limiting reactant: Gas stoichiometry helps identify the limiting reactant, which is the reactant that is completely consumed in a reaction, and determines the maximum amount of product that can be formed.
Overall, gas stoichiometry plays a crucial role in understanding and predicting the behavior of gases in chemical reactions. It is an essential tool for chemists in various fields, such as industrial processes, environmental studies, and pharmaceutical research.
Why is Gas Stoichiometry Important?
Gas stoichiometry is an essential concept in chemistry that allows us to quantify and predict the amounts of reactants and products involved in chemical reactions involving gases. By understanding and applying gas stoichiometry, chemists can calculate the theoretical yields of products, determine the limiting reactant, and optimize reaction conditions.
One of the main reasons why gas stoichiometry is important is its practical applications in various industries. For example, in the field of environmental science, gas stoichiometry is used to study air pollution and analyze the emissions from factories and vehicles. It helps scientists to determine the quantity and composition of pollutants released into the atmosphere, which can then be used to develop effective strategies for reducing air pollution and improving air quality.
In addition, gas stoichiometry is crucial in the field of industrial chemistry, where it is used to design and optimize chemical processes. For instance, in the production of ammonia, an important chemical used in fertilizers and other applications, gas stoichiometry is used to determine the optimal ratio of reactants to achieve the highest yield of ammonia. It also helps to identify any side reactions or unwanted byproducts that may occur during the process.
Furthermore, gas stoichiometry plays a vital role in understanding and analyzing the behavior of gases in various systems, such as in combustion reactions, gas phase equilibrium, and the behavior of gases in confined spaces. This knowledge is essential in fields such as atmospheric science, where the understanding of gas stoichiometry is necessary for predicting and studying the behavior of gases in the atmosphere, including processes related to climate change.
In conclusion, gas stoichiometry is a fundamental concept in chemistry with important practical applications in various fields. It allows scientists to quantify and predict the amounts of reactants and products involved in chemical reactions, which is essential for understanding and optimizing chemical processes, studying air pollution, and analyzing the behavior of gases in different systems. Without gas stoichiometry, it would be difficult to accurately predict and control the outcomes of chemical reactions involving gases.
How to Solve Gas Stoichiometry Problems?

Gas stoichiometry is the study of the relationship between the amounts of reactants and products in a chemical reaction involving gases. It allows us to calculate the quantities of gases involved in a reaction using balanced chemical equations and known data, such as the volume, pressure, and temperature of the gases. Solving gas stoichiometry problems involves a series of steps that help us determine the unknown quantities of gases.
To solve gas stoichiometry problems, follow these steps:
- Write the balanced chemical equation for the reaction. This equation provides the ratio of reactants to products.
- Convert the given information into the appropriate units. For example, if the given information is in volume, convert it to moles using the ideal gas law.
- Use the stoichiometry of the balanced equation to determine the ratio of moles of the given substance to the desired substance.
- Apply the given ratio to the converted value to calculate the moles of the desired substance.
- Convert the moles of the desired substance into the desired unit, such as mass or volume, using the molar mass or ideal gas law.
By following these steps, you can solve gas stoichiometry problems and determine the quantities of gases involved in a chemical reaction. It is important to remember to use the correct units and conversion factors throughout the calculations to obtain accurate results.
Key Concepts in Gas Stoichiometry
Gas stoichiometry involves the study of the relationships between the quantities of reactants and products in a chemical reaction, specifically those involving gases. It allows us to determine the amount of gas produced or consumed in a reaction based on the stoichiometric coefficients in the balanced chemical equation.
Mole-to-Mole Ratio: The mole-to-mole ratio is a key concept in gas stoichiometry. It is derived from the balanced chemical equation and represents the ratio of the number of moles of one substance to the number of moles of another substance in a reaction. This ratio is used to convert between the amounts of reactants and products involved in the reaction.
Gas Laws: Gas stoichiometry is closely linked to the fundamental gas laws, such as Boyle’s Law, Charles’s Law, and Avogadro’s Law. These laws describe the behavior of gases under different conditions of temperature, pressure, and volume. Understanding these laws is crucial for accurately calculating gas stoichiometry problems.
Stoichiometric Calculations: Gas stoichiometry involves performing calculations to determine the quantity of gas involved in a chemical reaction. This can include determining the volume, pressure, or number of moles of gas produced or consumed. These calculations are based on the principles of stoichiometry and the given information about the reaction.
Gas Density: Gas density is another important concept in gas stoichiometry. It is defined as the mass of a gas divided by its volume. Knowledge of gas density allows us to determine the mass or volume of a gas under specific conditions, which is necessary for stoichiometric calculations.
- Overall, gas stoichiometry involves understanding the mole-to-mole ratios, gas laws, stoichiometric calculations, and gas density in order to determine the quantities of reactants and products involved in a chemical reaction.
Steps to Solve a Gas Stoichiometry Problem
Gas stoichiometry problems involve calculations related to the amount of reactants and products involved in a chemical reaction, specifically in the gas phase. These problems are often solved using the ideal gas law, which relates the pressure, volume, temperature, and moles of a gas.
To solve a gas stoichiometry problem, you can follow a series of steps:
- Write the balanced chemical equation: Start by writing the balanced chemical equation for the reaction. This equation shows the reactants and products involved, as well as their stoichiometric coefficients.
- Convert units, if necessary: If the given information is not in the desired units (e.g., moles), convert it using appropriate conversion factors. Make sure to use the correct molar masses for the conversion.
- Determine the known and unknown quantities: Identify the known and unknown quantities in the problem. The known quantities are the values given in the question, while the unknown quantity is what you need to calculate.
- Apply the ideal gas law, if applicable: If the problem involves gases, apply the ideal gas law equation: PV = nRT. This equation relates the pressure, volume, temperature, and moles of a gas. Rearrange the equation to solve for the unknown quantity.
- Use stoichiometry: If the problem involves a chemical reaction, use stoichiometry to relate the moles of the known and unknown substances. Use the stoichiometric coefficients from the balanced equation to determine the mole ratios.
- Perform the calculations: Substitute the known values into the appropriate equations and solve for the unknown quantity. Make sure to use correct significant figures and units in the final answer.
- Check your answer: Finally, double-check your calculations and ensure that your answer makes sense in the context of the problem. If necessary, round your answer to the appropriate number of significant figures.
By following these steps, you can successfully solve gas stoichiometry problems and determine the quantities of reactants and products involved in a chemical reaction.
Sample Gas Stoichiometry Problems

In gas stoichiometry, we use the principles of stoichiometry to calculate the quantities of gases involved in a chemical reaction. This practice is crucial for understanding and predicting the outcomes of various chemical reactions. Let’s take a look at some sample gas stoichiometry problems to further illustrate its application.
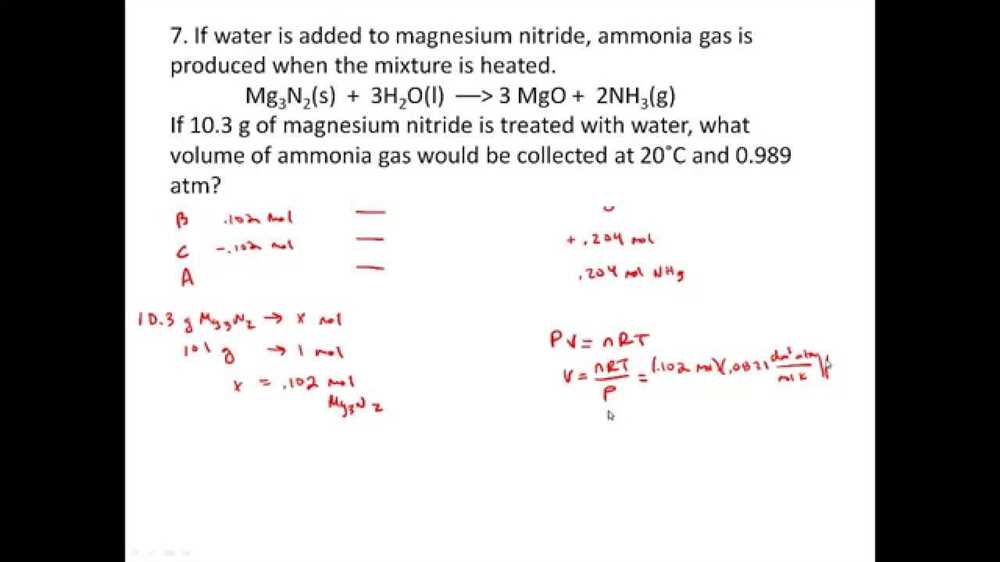
Example 1: What volume of nitrogen gas (N2) is needed to react completely with 24 liters of hydrogen gas (H2) to produce ammonia gas (NH3)?
Solution:
- Write the balanced chemical equation for the reaction: N2 + 3H2 → 2NH3.
- Convert the given volume of hydrogen gas (H2) to moles using the ideal gas law.
- Use the stoichiometric ratio between hydrogen gas (H2) and nitrogen gas (N2) to determine the moles of nitrogen gas needed.
- Convert the moles of nitrogen gas to volume using the ideal gas law.
Example 2: A sample of magnesium metal (Mg) reacts with hydrochloric acid (HCl) to produce hydrogen gas (H2). If the volume of hydrogen gas collected over water at a certain temperature and pressure is 5.6 liters, what mass of magnesium metal was used?
Solution:
- Write the balanced chemical equation for the reaction: Mg + 2HCl → MgCl2 + H2.
- Convert the given volume of hydrogen gas (H2) to moles using the ideal gas law.
- Use the stoichiometric ratio between hydrogen gas (H2) and magnesium metal (Mg) to determine the moles of magnesium used.
- Convert the moles of magnesium metal to mass using the molar mass of magnesium.
By practicing these sample gas stoichiometry problems, you can develop a better understanding of how to apply gas stoichiometry principles to solve various chemical reactions. Remember to always balance the chemical equation and use the appropriate stoichiometric ratios to accurately calculate the quantities of gases involved.