In the world of chemical analysis, the flame test is a crucial technique used to identify the presence of various elements in a sample. A flame test kit is an essential tool for this process, offering scientists and researchers a portable and convenient way to perform these tests in the laboratory or in the field.
The flame test kit typically consists of a set of different metal salts, such as sodium chloride, potassium chloride, and calcium chloride, each of which produces a characteristic color when exposed to a flame. By heating a sample with a flame and observing the color of the resulting flame, scientists can identify which elements are present in the sample.
Using a flame test kit is a relatively simple process. A small amount of the powdered salt is usually placed on a metal loop or wire, which is then heated in a Bunsen burner flame. The color of the flame that is emitted can then be compared to a reference chart to determine the presence of specific elements.
Flame test kits are used in a wide range of scientific disciplines, including chemistry, geology, and forensic science. They are particularly useful for determining the presence of metals in a sample, making them invaluable tools for identifying unknown substances or confirming the composition of known compounds.
In conclusion, a flame test kit is an indispensable tool for chemical analysis, allowing scientists and researchers to quickly and easily identify the presence of specific elements in a sample. With its portability and simplicity of use, this tool has become a staple in laboratories around the world, enabling important discoveries and advancements in various scientific fields.
What is a Flame Test Kit?
A flame test kit is a set of tools and chemicals used to identify the presence of certain elements in a substance by observing the color of the flame produced when it is subjected to high heat. This type of test is based on the principle that different elements emit unique colors of light when heated, allowing for their identification.
Typically, a flame test kit includes a variety of metal salts, such as potassium chloride, lithium chloride, and sodium chloride, which are known to produce distinct flame colors. These salts are mixed with a solvent to create a solution that can be applied to a sample for testing. The solution is then heated using a Bunsen burner or similar heat source, and the resulting flame color is observed.
The flame colors produced during a flame test are characteristic to specific elements. For example, potassium ions produce a violet flame, while sodium ions produce a yellow flame. By comparing the observed flame color to a reference chart or database, it is possible to determine which element is present in the substance being tested. This information can be valuable in various fields, such as chemistry, forensic science, and environmental analysis.
Benefits of a Flame Test Kit
Using a flame test kit offers several benefits. One of the main advantages is its simplicity and ease of use. Flame tests can be conducted relatively quickly and require minimal equipment, making them accessible to a wide range of users, including students, researchers, and professionals.
Moreover, flame tests are non-destructive, meaning that they do not alter or damage the sample being tested. This is particularly useful when working with limited or valuable samples. Additionally, flame tests are often cost-effective compared to other analytical techniques, making them a cost-efficient method for preliminary identification of elements.
Overall, a flame test kit provides a convenient and reliable way to identify the presence of certain elements based on their unique flame colors. Whether it’s in a laboratory setting or during a classroom experiment, a flame test kit can be a valuable tool for anyone working with elemental analysis.
How Does a Flame Test Kit Work?
A flame test kit is a tool used to identify the presence of certain elements in a sample based on the color of the flame that is produced when the sample is burned. This test relies on the principle that different elements emit specific colors when they are heated in a flame. By observing the color of the flame, scientists can determine which elements are present in the sample.
The flame test kit consists of a set of different metal salts that are used as reagents. These metal salts are usually in solid form and can be dissolved in a liquid solvent. The kit also includes a burner or a Bunsen burner, which is used to heat the sample. To perform a flame test, a small amount of the sample is usually moistened with the reagent and then placed in the flame. The color of the flame that is produced is then observed and compared to a chart of known flame colors to identify the elements present.
The color of the flame that is produced during a flame test is due to the emission of light by the excited electrons in the atoms of the element. When the sample is heated, the electrons in the atoms absorb energy and move to higher energy levels. As the electrons return to their original energy levels, they release energy in the form of light. Each element has a unique arrangement of electrons and energy levels, resulting in the emission of specific colors of light.
The flame test kit is a simple and affordable tool that is widely used in chemistry laboratories and educational settings. It is especially useful for identifying certain elements that are commonly found in inorganic compounds, such as sodium, potassium, calcium, and copper. However, it is important to note that the flame test is not a definitive method for identifying elements and should be used in conjunction with other analytical techniques for accurate results.
The Importance of Flame Tests in Chemistry
Flame tests are an essential technique in chemistry used to identify the presence of certain elements in a compound. By heating a substance in a flame, we can observe the characteristic color that is emitted, which corresponds to specific elements in the compound. Flame tests provide a quick and easy way to determine the elemental composition of unknown substances, making them an invaluable tool in chemical analysis.
One of the key advantages of flame tests is their simplicity and accessibility. They require minimal equipment, making them suitable for both educational and laboratory settings. By using a simple flame test kit, which typically includes a wire loop and various metal salts, chemists can easily perform these tests to gain valuable insights into the composition of a substance. This makes flame tests an ideal technique for introductory chemistry courses, as they provide a hands-on approach to learning about chemical elements.
Moreover, flame tests are highly specific and sensitive, allowing for the identification of even trace amounts of certain elements. Each element emits a unique color when exposed to a flame, allowing chemists to distinguish between different elements in a sample. This specificity is crucial in fields such as forensic science, where the identification of trace elements can provide crucial evidence in solving crimes. Flame tests are also commonly used in environmental analysis to detect the presence of pollutants, helping to monitor and assess the impact of human activities on ecosystems.
In conclusion, flame tests play a critical role in chemistry by providing a simple yet effective method for identifying elements in compounds. Their accessibility, specificity, and sensitivity make them an indispensable tool in various fields of study. Whether in educational settings or professional laboratories, flame tests continue to contribute to our understanding of chemical composition and the world around us.
Different Types of Flame Test Kits
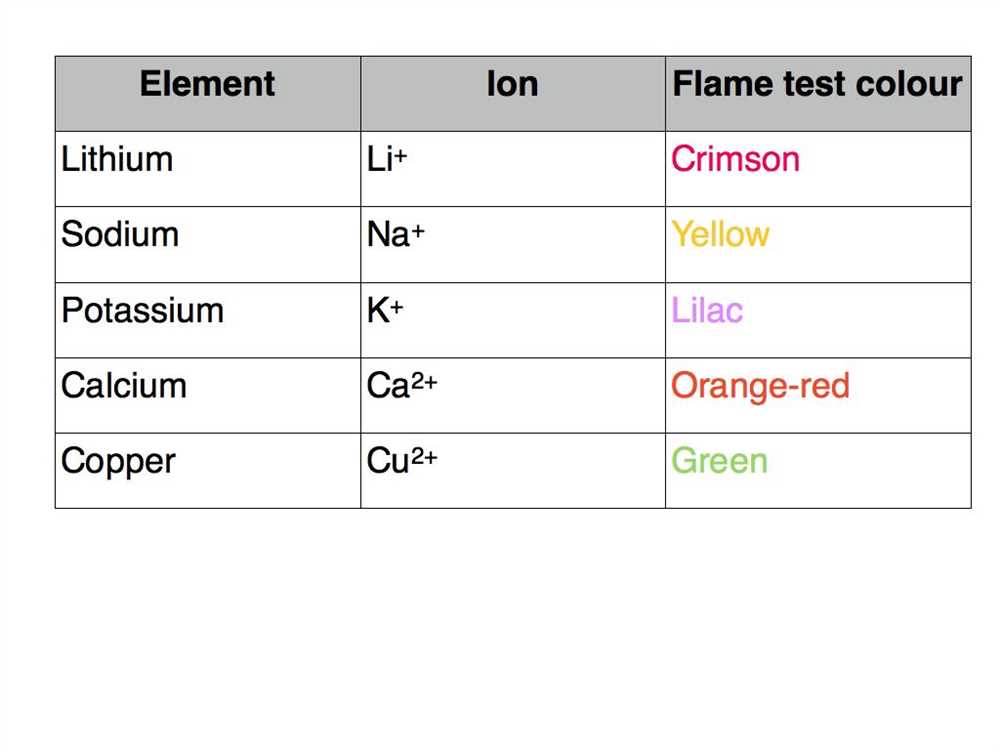
A flame test kit is a laboratory tool used to identify the presence of certain metal ions in a compound based on the characteristic color emitted by the metal ion when it is heated in a flame. Different types of flame test kits are available, each offering specific features and capabilities.
1. Basic Flame Test Kit: The basic flame test kit usually consists of a Bunsen burner, a set of metal wire loops, and metal salts of different elements. The metal salts are dipped into the flame, and the color emitted is observed and compared to a reference chart to identify the presence of specific metals.
2. Advanced Flame Test Kit: An advanced flame test kit may include additional accessories such as safety goggles, a flame-resistant mat, and a set of metal salts for a wider range of elements. It may also come with a more precise temperature control feature to ensure accurate results.
3. Portable Flame Test Kit: Portable flame test kits are designed for field use or situations where a laboratory setting is not available. These kits are compact and lightweight, often including a portable Bunsen burner or a handheld flame source and a set of metal salts in small vials. They are convenient for on-site testing and can be easily transported.
4. Spectrometer Flame Test Kit: A spectrometer flame test kit offers more advanced capabilities. It includes a spectrometer device that measures the wavelengths of light emitted by the metal ions when heated in a flame. This allows for accurate identification and quantification of the metal ions present in a compound.
5. Educational Flame Test Kit: Designed specifically for educational purposes, an educational flame test kit often includes simplified instructions and materials suitable for students. It may come with pre-made metal wire loops and a limited set of metal salts for common elements, allowing students to learn about flame tests and the colors associated with different metal ions.
In conclusion, flame test kits come in various types and cater to different needs. Whether for basic identification purposes, advanced analysis, portability, or educational use, there is a flame test kit available for every application.
How to Use a Flame Test Kit
A flame test kit is a tool used in chemistry to identify the presence of certain elements in a substance by observing the color of the flame produced when the substance is heated. The kit usually contains a set of chemical compounds, each of which produces a unique color when burned.
To use a flame test kit, follow these steps:
1. Gather the necessary materials:
- A Bunsen burner or a similar heat source
- A flame test wire or loop
- The substance to be tested
- Chemicals from the flame test kit
- Safety goggles and gloves
2. Prepare the flame test wire:
Clean the flame test wire or loop by dipping it in hydrochloric acid and then rinsing it with distilled water. This step is important to ensure accurate results and prevent contamination.
3. Dip the flame test wire into the substance:
Dip the cleaned flame test wire into the substance to be tested. Make sure to coat the wire evenly with a small amount of the substance.
4. Heat the flame test wire:
Hold the coated flame test wire in the flame of the Bunsen burner or heat source. Observe the color of the flame produced. The color will be characteristic of the specific element present in the substance.
5. Compare the observed flame color:
Compare the observed flame color with the colors produced by the chemicals in the flame test kit. Identify the element present in the substance based on the matching flame color.
Note: It is important to perform the flame test in a well-ventilated area and take necessary safety precautions to avoid any accidents or inhalation of harmful fumes.
By following these steps, you can effectively use a flame test kit to identify the presence of specific elements in a substance.
Safety Precautions When Using a Flame Test Kit
Using a flame test kit can be an exciting and educational experience, but it is important to prioritize safety when conducting experiments. The following precautions should be taken to ensure a safe and controlled environment:
- Wear appropriate protective gear: When working with a flame test kit, it is essential to wear safety goggles and heat-resistant gloves to prevent any accidents or injuries. These protective measures will shield your eyes and hands from potential hazards.
- Work in a well-ventilated area: The flame test kit involves burning chemicals, which can produce fumes or smoke. It is crucial to conduct experiments in a well-ventilated area to prevent the buildup of toxic gases. Open windows or use a fume hood if available.
- Keep flammable materials away: Before starting the experiment, make sure to clear the area of any flammable materials or substances. Keep chemicals, solvents, and other potentially hazardous items away from the open flame to reduce the risk of fire.
- Use a stable surface: Set up the flame test kit on a stable and heat-resistant surface. This will prevent any accidental spills or tip-overs that could lead to injuries or damage.
- Avoid overcrowding: Only work with one sample at a time, ensuring that there is sufficient space between each sample and the flame. Overcrowding the samples can increase the risk of cross-contamination or accidental contact with the flame.
- Be cautious with chemicals: Handle the chemicals used in the flame test kit with care. Follow the instructions provided, and avoid touching or ingesting any substances. Dispose of chemicals properly after use to prevent contamination or harm.
By following these safety precautions, you can maximize the educational value of using a flame test kit while minimizing potential risks. Always remember to prioritize safety and take necessary precautions when working with open flames and chemicals.
Common Applications of Flame Test Kits
Flame test kits are widely used in various industries and scientific research settings for their ability to identify and determine the presence of different metal ions. These test kits utilize the unique emission spectra produced by heated metals when introduced to a flame to accurately identify the elements present in a sample.
Chemical analysis: The most common application of flame test kits is in chemical analysis. By subjecting a sample to a flame, the emitted light is observed through the use of filters or spectrometers to identify the specific metal ions present. This information can be crucial in determining the composition and purity of various substances, such as identifying impurities or confirming the presence of specific elements in pharmaceuticals, minerals, or unknown samples.
Metallurgical research: Flame test kits are used extensively in metallurgical research to identify and analyze different metal alloys. By subjecting the alloy to a flame, researchers can determine the composition and proportions of different metals present in the sample. This information is vital in understanding the properties and behavior of various alloys and can aid in the development of new materials with desired characteristics, such as increased strength or corrosion resistance.
Forensic analysis: Flame test kits are also employed in forensic analysis to aid in investigations and crime scene analysis. By performing flame tests on suspected substances or residues, scientists can identify the presence of specific elements that may be related to a crime. This information can help establish links between suspects, victims, or crime scenes and provide valuable evidence in criminal proceedings.
Education and demonstration: Flame test kits are widely used in educational settings to teach students about the properties of different elements and how they can be identified using flame tests. These kits provide a hands-on approach to learning and allow students to witness the distinct colors and characteristics associated with different metal ions. They also serve as a captivating demonstration tool in science classrooms and public events, showcasing the fascinating world of chemistry and the unique behavior of elements when subjected to heat.