Understanding the concepts of matter and change is fundamental to the study of chemistry. In Chapter 1, we explore the properties of matter and how it can undergo various changes. In this article, we will provide answers to the worksheet questions from Chapter 1, helping you to better grasp these concepts.
One of the key topics covered in Chapter 1 is the classification of matter. We will discuss the different types of matter, including elements, compounds, and mixtures, and explain how to distinguish between them. By understanding the composition and properties of different types of matter, you will be able to answer the worksheet questions with confidence.
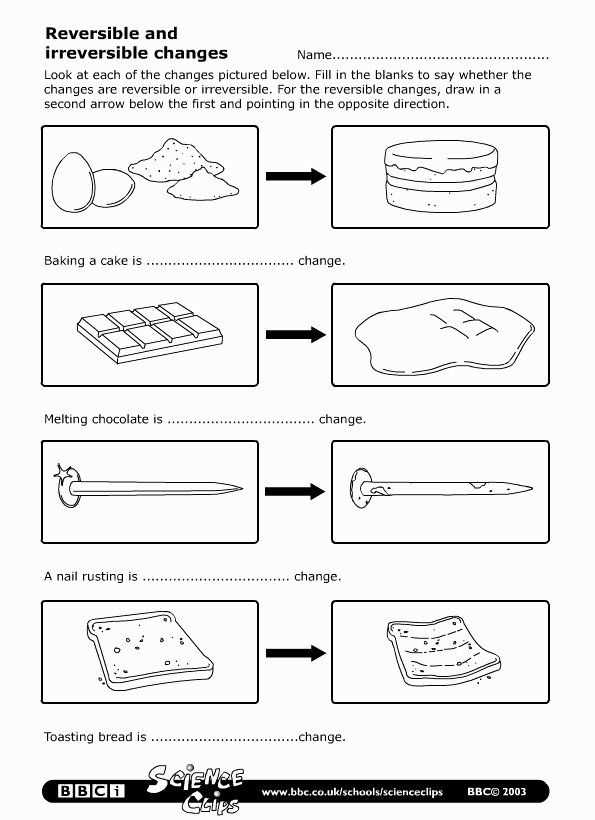
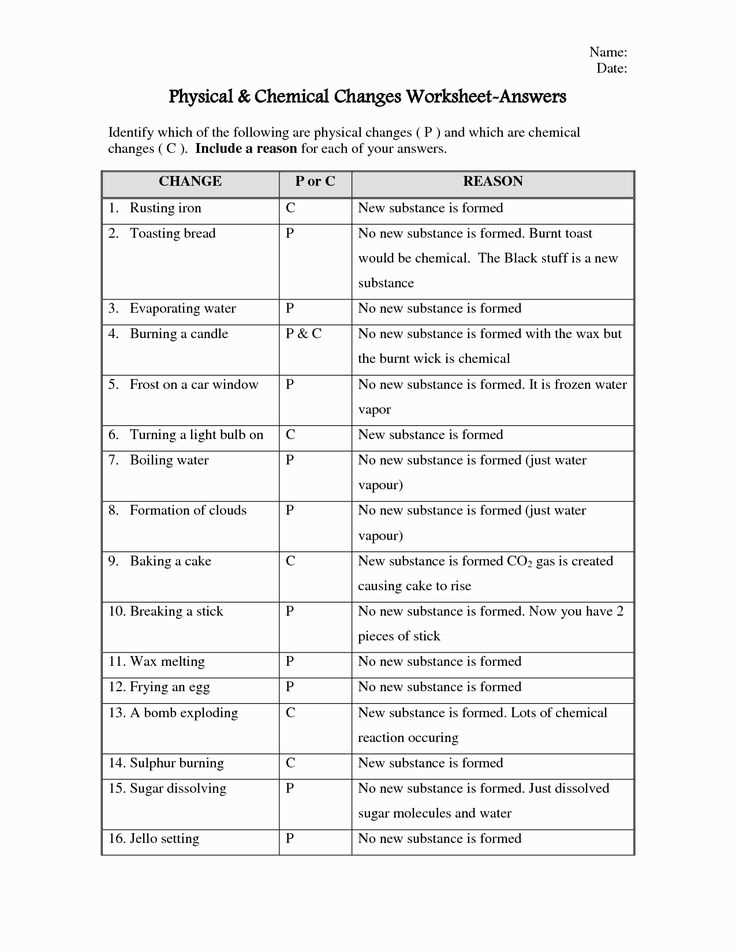
Another important aspect of Chapter 1 is the concept of physical and chemical changes. We will explore the differences between these two types of changes and provide examples to help solidify your understanding. By understanding the signs and outcomes of physical and chemical changes, you will be able to accurately answer the worksheet questions and apply this knowledge to real-world situations.
Chapter 1 Matter and Change Worksheet Answers

In Chapter 1 of the Matter and Change textbook, students are introduced to the fundamental concepts of matter and its properties. The chapter explores topics such as the classification of matter, physical and chemical changes, and measurements in chemistry. To reinforce their understanding of these concepts, students are often assigned worksheet questions.
One of the key aspects of the chapter is the classification of matter. Students learn about the different types of matter, including pure substances and mixtures. They also explore the various ways in which matter can be classified, such as by its physical state (solid, liquid, or gas) or by its composition (elements or compounds).
Another important concept covered in Chapter 1 is physical and chemical changes. Students learn to distinguish between these two types of changes and understand the principles behind each. They learn that physical changes do not alter the composition of a substance, while chemical changes result in the formation of new substances with different properties.
The chapter also delves into measurements in chemistry. Students learn about the International System of Units (SI) and its importance in scientific measurements. They explore different measurement tools and techniques, such as balances and graduated cylinders, and learn to use them effectively in the laboratory.
In the worksheet answers for Chapter 1, students can expect to find explanations and solutions to various questions related to these concepts. The answers will help them solidify their understanding of the material covered in the chapter and provide guidance for further study.
Understanding the Properties of Matter
Matter is the physical substance that makes up everything in the universe. It is composed of atoms and molecules, which are constantly in motion. Understanding the properties of matter is crucial in various scientific fields, including chemistry and physics.
One important property of matter is its physical state, which can be solid, liquid, or gas. Solids have a fixed shape and volume, while liquids have a definite volume but take the shape of their container. Gases, on the other hand, have neither a fixed shape nor volume and can expand to fill any container. The physical state of matter can be changed through heating or cooling, resulting in changes in the arrangement and movement of its particles.
Another property of matter is its density, which is the measure of mass per unit volume. Density can be used to determine whether an object will float or sink in a fluid. Objects with a higher density than the fluid they are placed in will sink, while objects with a lower density will float. Density is also related to the packing of particles in a substance. Substances with particles tightly packed together have a higher density, while those with particles more spread out have a lower density.
- Chemical properties of matter refer to its ability to undergo chemical reactions and form new substances. This involves changes in the chemical composition of matter, resulting in the formation of new bonds and the release or absorption of energy. Chemical properties can include flammability, reactivity with other substances, and the ability to undergo oxidation or reduction reactions.
- Physical properties of matter are those that can be observed or measured without changing the composition of the substance. These include properties like color, odor, taste, texture, melting point, boiling point, and conductivity. Physical properties can be used to identify substances and to classify them into different categories.
- Matter can also have intensive and extensive properties. Intensive properties do not depend on the amount of matter present and include properties like density, temperature, and color. Extensive properties, on the other hand, depend on the amount of matter and include properties like mass and volume.
Overall, understanding the properties of matter is essential for scientists to study and manipulate substances in various fields ranging from medicine to materials science. By uncovering the characteristics and behaviors of matter, we can gain a deeper understanding of the world around us and develop new technologies and applications.
Exploring Chemical and Physical Changes
In the study of matter and its properties, it is important to understand the concepts of chemical and physical changes. Chemical changes involve the formation of new substances with different chemical properties, while physical changes involve alterations in the state or appearance of matter, without changing its chemical composition. Both types of changes are essential to our understanding of the universe and are constantly occurring in our everyday lives.
Chemical changes occur when two or more substances react with each other to form new substances. This can be observed in processes such as combustion, where a fuel combines with oxygen to produce heat and light, or in the rusting of iron, where iron reacts with oxygen in the presence of water to form iron oxide. These reactions involve the breaking and forming of chemical bonds, resulting in the creation of new substances with unique properties.
- Combustion: The process of a substance reacting with oxygen to produce heat and light.
- Rusting: The process of iron reacting with oxygen and water to form iron oxide.
Physical changes, on the other hand, involve alterations in the physical properties of matter, such as its shape, size, or state of matter. This can be seen in processes such as melting, where a solid substance changes into a liquid, or evaporation, where a liquid changes into a gas. These changes do not involve the formation of new substances, but rather a rearrangement of the existing particles within the material.
- Melting: The process of a solid substance changing into a liquid due to an increase in temperature.
- Evaporation: The process of a liquid substance changing into a gas due to an increase in temperature.
By studying chemical and physical changes, scientists are able to understand the fundamental properties of matter and how it behaves under different conditions. This knowledge is crucial in fields such as chemistry, materials science, and engineering, where the manipulation and control of matter is essential in the development of new technologies and advancements. Whether it is the creation of new compounds or the understanding of phase changes, exploring chemical and physical changes allows us to unlock a deeper understanding of the world around us.
Classifying Matter
Classifying matter is an essential part of understanding the physical world around us. Matter can be classified in various ways, depending on its composition and properties. By categorizing matter, scientists are able to study and analyze different substances, as well as predict their behavior and reactions.
Composition: One way to classify matter is based on its composition. Matter can be classified as either pure substances or mixtures. Pure substances are made up of only one type of particle and cannot be separated into simpler substances by physical means. Examples of pure substances include elements and compounds. Elements are substances that cannot be broken down into simpler substances and are represented by their atomic symbols. Compounds, on the other hand, are made up of two or more elements chemically combined in fixed proportions.
Properties: Another way to classify matter is based on its physical and chemical properties. Physical properties are characteristics that can be observed or measured without changing the substance’s composition. Examples of physical properties include color, density, boiling point, and conductivity. Chemical properties, on the other hand, describe how a substance can change or react to form new substances. Examples of chemical properties include flammability, reactivity, and toxicity.
In addition to classifying matter based on composition and properties, matter can also be classified further into more specific categories. These include homogeneous and heterogeneous mixtures, as well as colloids and suspensions. Homogeneous mixtures are uniform in composition and have the same properties throughout, while heterogeneous mixtures have different compositions and properties in different areas. Colloids are mixtures in which particles are dispersed throughout another substance, and suspensions are mixtures in which particles settle out over time.
By understanding and classifying matter, scientists are able to study and manipulate substances more effectively. Whether it is analyzing the composition of a rock or developing new materials for various applications, the classification of matter plays a crucial role in scientific research and everyday life.
Elements and Compounds

Elements and compounds are two types of pure substances that make up everything in the universe. They are made up of atoms, which are the smallest units of matter. Each element is made up of only one type of atom, while compounds are made up of two or more different types of atoms chemically combined together.
Elements are the building blocks of matter. There are currently 118 known elements, which are organized in the periodic table. Each element has a unique set of properties, such as atomic number, symbol, and atomic mass. Some common examples of elements include hydrogen, oxygen, carbon, and gold.
Compounds, on the other hand, are substances that are formed when atoms of different elements chemically combine. The atoms in a compound are held together by chemical bonds. Compounds have unique properties that are different from the elements they are made of. For example, water (H2O) is a compound made up of two hydrogen atoms and one oxygen atom. It has different properties compared to hydrogen and oxygen alone.
In summary, elements are pure substances made up of only one type of atom, while compounds are made up of two or more different types of atoms chemically bonded together. Elements and compounds play a crucial role in understanding the composition and properties of matter.
The Periodic Table and Atomic Structure
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It provides a systematic way to categorize and understand the properties of different elements. The periodic table is divided into vertical columns called groups or families, and horizontal rows called periods.
Each element in the periodic table is represented by a unique symbol, usually consisting of one or two letters. The atomic number of an element represents the number of protons in the nucleus of its atoms. The number of protons determines the identity of the element, as each element has a unique atomic number. The atomic mass of an element is the weighted average mass of its isotopes.
The atomic structure of an element refers to the arrangement of particles within its atom. Atoms are composed of a central nucleus, which contains protons and neutrons, surrounded by a cloud of electrons. The protons are positively charged, while the electrons are negatively charged. The neutrons have no charge and are responsible for adding mass to the atom.
Each element has a specific number of electrons that occupy different energy levels or shells around the nucleus. The electron configuration of an element describes the distribution of electrons in these energy levels. The distribution of electrons determines the chemical behavior of an element and its ability to form bonds with other elements.
The periodic table provides valuable information about the properties and behavior of elements. It allows scientists to predict the reactivity, valence electron configuration, and chemical properties of an element based on its position in the table. The periodic table is a fundamental tool in chemistry, enabling scientists to understand and manipulate the building blocks of matter.
Chemical Formulas and Equations
Chemical formulas are a way of representing the composition of substances. They use symbols to represent the elements that make up a compound and indicate the ratio of atoms in the compound. For example, the formula for water is H2O, which shows that each water molecule consists of two hydrogen atoms bonded to one oxygen atom. Chemical formulas are concise and standardized, allowing scientists to easily communicate and understand the composition of different substances.
In chemical equations, chemical formulas are used to represent the reactants and products of a chemical reaction. A chemical equation shows the chemical change that occurs during a reaction. It consists of chemical formulas separated by arrows, with the reactants on the left side and the products on the right side. The arrow indicates the direction of the reaction, showing the conversion of reactants into products. The coefficients in front of the formulas indicate the relative amounts of each substance involved in the reaction.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants must equal the total mass of the products. Chemical equations are balanced to ensure that this law is upheld. Balancing a chemical equation involves adjusting the coefficients so that the number of atoms of each element is the same on both sides of the equation. This allows for an accurate representation of the reaction and ensures that mass is conserved.
Key Points:
- Chemical formulas represent the composition of substances and indicate the ratio of atoms in compounds.
- Chemical equations show the chemical change that occurs during a reaction, with reactants on the left side and products on the right side.
- Balancing a chemical equation involves adjusting coefficients to ensure that the number of atoms of each element is the same on both sides of the equation.
- The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.