Isotopes, ions, and atoms are fundamental concepts in chemistry that help us understand the nature of matter. These concepts play a crucial role in determining the properties and behavior of elements and their compounds. To fully grasp these concepts, it is essential to have a clear understanding of subatomic particles – protons, neutrons, and electrons – and their role in creating different isotopes, ions, and atoms.
Protons, neutrons, and electrons are the building blocks of all matter. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. These particles are located in the nucleus of an atom. The number of protons defines an element, while the sum of protons and neutrons determines the mass of an atom.
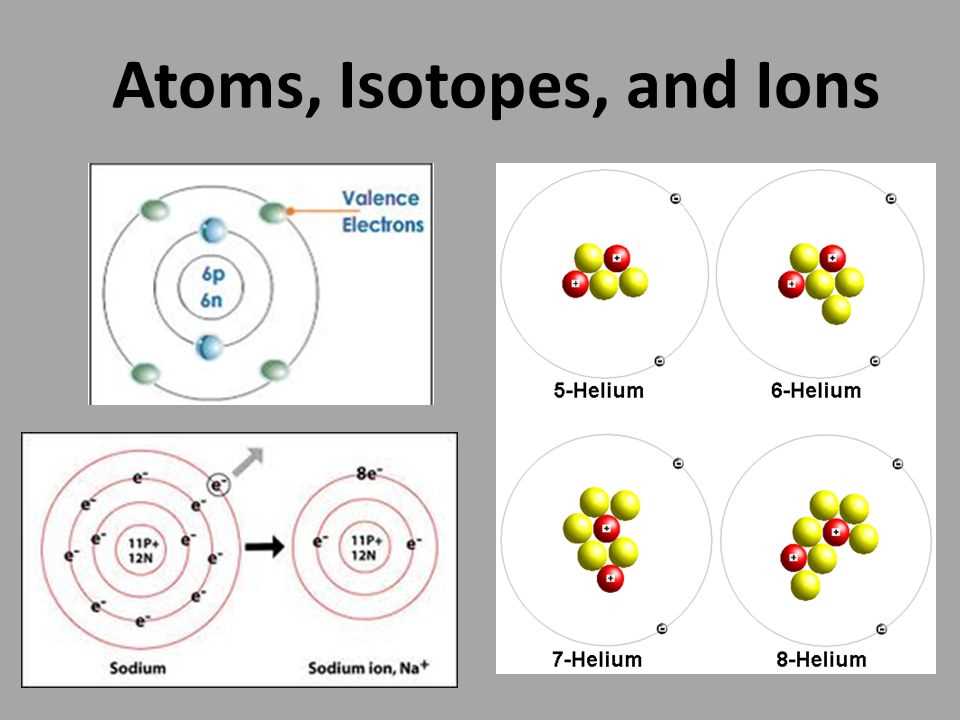
When we talk about isotopes, we refer to atoms of the same element that have different numbers of neutrons. This means isotopes have the same number of protons but different masses. Isotopes of an element share similar chemical properties but may have different physical properties due to their varying weights.
Ions, on the other hand, are atoms or groups of atoms that have gained or lost electrons, resulting in a net charge. When an atom loses an electron, it becomes a positively charged ion, called a cation. Conversely, when an atom gains an electron, it becomes a negatively charged ion, known as an anion. These charged ions play a crucial role in chemical reactions and the formation of compounds.
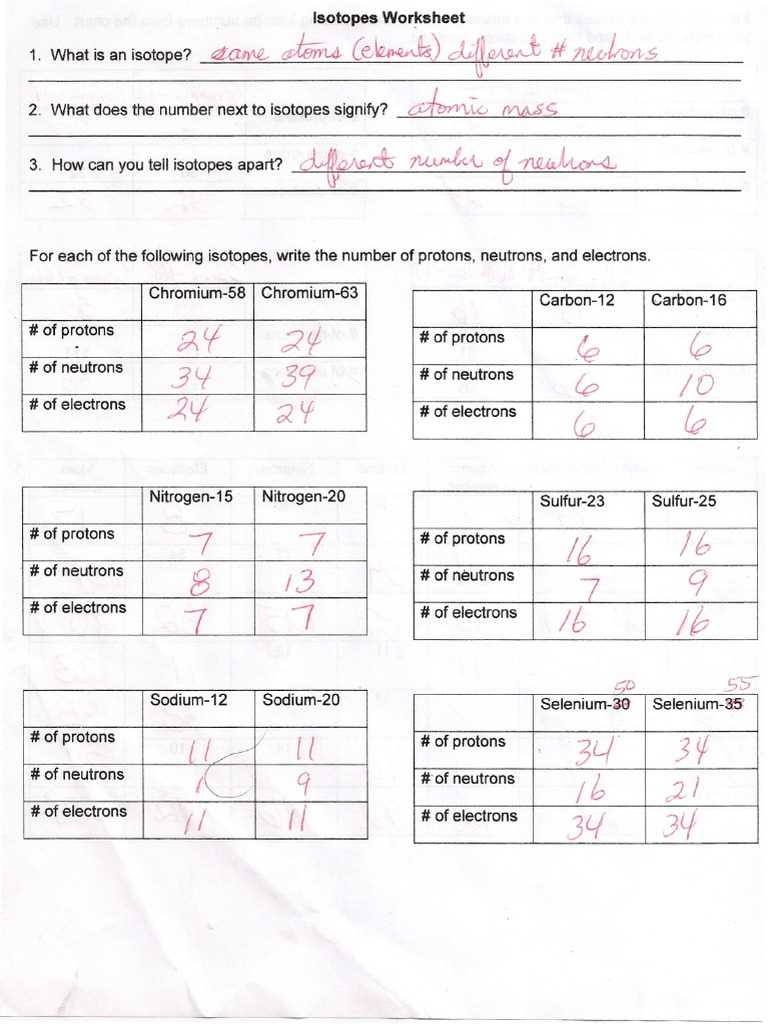
What are isotopes?
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. They are atoms of the same element that have different masses. Isotopes can be found for almost every element in the periodic table.
Definition: Isotopes are atoms that have the same atomic number (number of protons) but different atomic masses (sum of protons and neutrons). They are identified by their mass number, which represents the total number of protons and neutrons in the nucleus of an atom. For example, carbon-12 and carbon-14 are two isotopes of the element carbon.
Isotopes exhibit similar chemical properties since their atomic number (protons) remains the same. However, they may have different physical properties, such as boiling points and densities, due to variations in their atomic masses.
- Isotopes are represented using the element’s symbol followed by a hyphen and the mass number. For example, carbon-12 is represented as C-12 and carbon-14 as C-14.
- Isotopes can be stable or radioactive. Stable isotopes do not undergo radioactive decay, while radioactive isotopes are unstable and decay over time, emitting radiation.
- Isotopes have various applications in fields such as medicine, archaeology, and environmental science. They can be used as tracers to track the movement of substances in biological systems or to determine the age of archaeological artifacts.
In conclusion, isotopes are variants of an element that have the same number of protons but different numbers of neutrons. They play a crucial role in understanding the behavior and properties of elements, as well as in various practical applications.
The concept of ions
Ions are electrically charged particles that are formed when atoms gain or lose electrons. Atoms are composed of protons, neutrons, and electrons. The protons have a positive charge, the neutrons have no charge, and the electrons have a negative charge. In a neutral atom, the number of protons equals the number of electrons, resulting in a balanced charge. However, when atoms gain or lose electrons, they become ions with a net positive or negative charge.
There are two types of ions: cations and anions. Cations are formed when atoms lose one or more electrons, resulting in a net positive charge. For example, if a sodium atom loses one electron, it becomes a sodium cation with a +1 charge. Anions, on the other hand, are formed when atoms gain one or more electrons, resulting in a net negative charge. For instance, if a chlorine atom gains one electron, it becomes a chloride anion with a -1 charge.
Ions play a crucial role in chemical reactions and the formation of compounds. They can bond with other ions or atoms through ionic bonds, which are formed by the attraction between oppositely charged ions. These bonds create ionic compounds, such as sodium chloride (NaCl), which is formed by the bonding of sodium cations and chlorine anions. The presence of ions also affects the physical and chemical properties of substances, such as their conductivity and solubility.
In summary, ions are electrically charged particles that are formed when atoms gain or lose electrons. They can be cations with a net positive charge or anions with a net negative charge. Ions play a vital role in chemical reactions and the formation of compounds through ionic bonds. Understanding the concept of ions is essential for studying various aspects of chemistry and its applications.
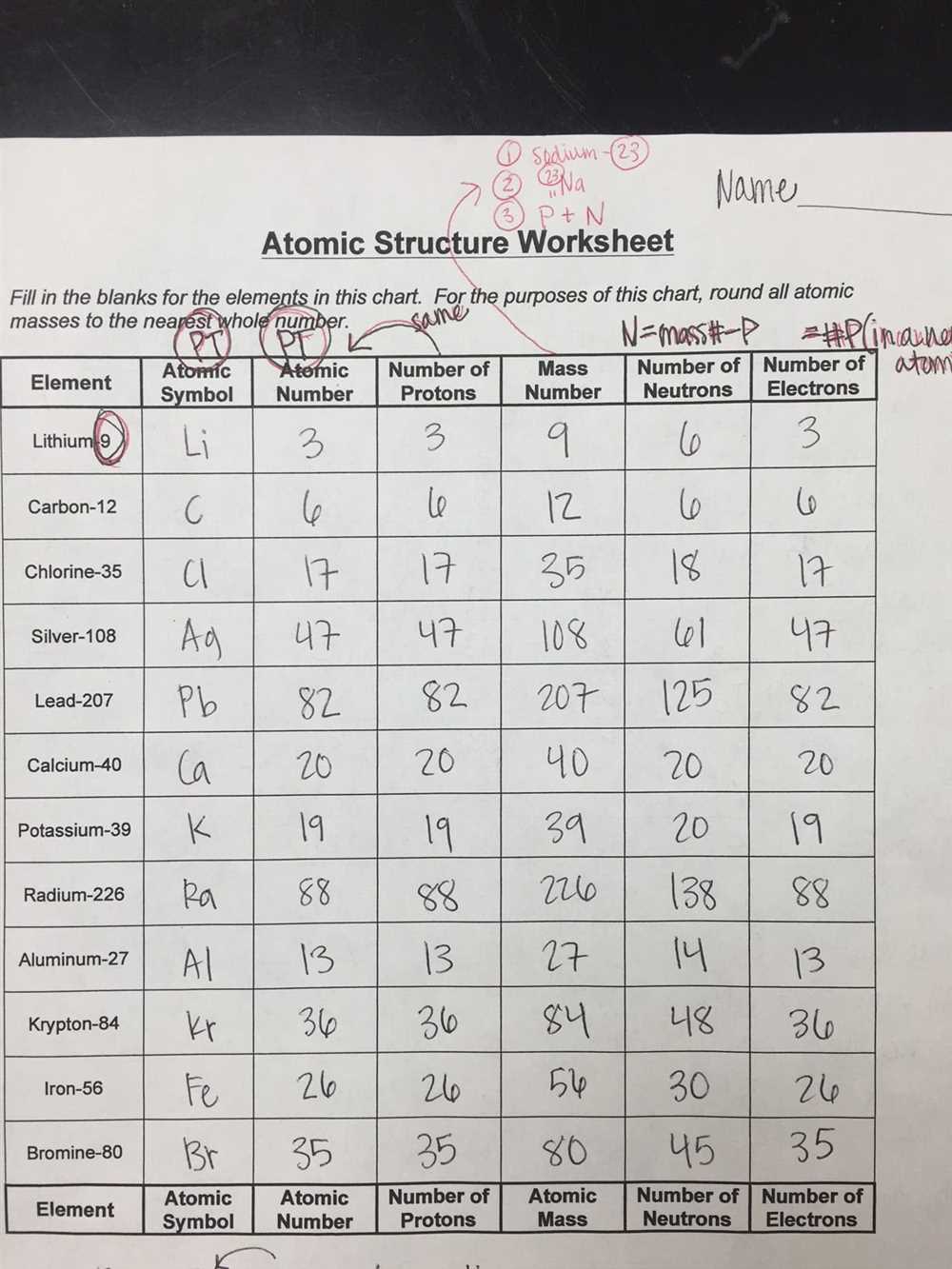
Understanding Atoms
Atomic Structure: Atoms are composed of three main subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit around the nucleus in specific energy levels or shells. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
Atom Identification: Each atom is characterized by its atomic number, which represents the number of protons it contains. For example, hydrogen has an atomic number of 1 because it has one proton, while carbon has an atomic number of 6 because it has six protons. The atomic mass of an atom is the sum of its protons and neutrons.
Isotopes: Isotopes are atoms of the same element that have a different number of neutrons. This means they have the same atomic number but different atomic masses. Isotopes can have various properties, such as different stabilities or radioactivity.
Ions: Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a positive or negative charge. When an atom loses an electron, it becomes a positively charged ion called a cation. On the other hand, when an atom gains an electron, it becomes a negatively charged ion called an anion.
Atomic Symbols and Notation: Atoms are commonly represented using atomic symbols, which consist of the element’s symbol and its atomic number. For example, carbon is represented as C, and its atomic number is 6, so its atomic symbol is written as ^12_6C. The superscript represents the atomic mass, and the subscript represents the atomic number.
Overall, understanding the fundamental properties and characteristics of atoms is essential for studying chemistry and delving into the complexities of the atomic world. Atoms are the building blocks that make up everything we see and touch, and their interactions with one another form the basis of chemical reactions and the diversity of matter.
Isotopes and their properties
The concept of isotopes refers to different forms of the same element that have the same number of protons but different numbers of neutrons in their nucleus. These variations in the number of neutrons result in isotopes with different atomic masses. For example, carbon, which has 6 protons in its nucleus, can have isotopes with 6, 7, or 8 neutrons, leading to carbon-12, carbon-13, and carbon-14 isotopes, respectively.
One of the key properties of isotopes is their atomic mass, which is the average mass of all the isotopes of an element. This value is often expressed as a decimal number on the periodic table. For example, the atomic mass of carbon is 12.01, which is a weighted average of the masses of carbon-12 and carbon-13 isotopes based on their natural abundance.
Isotopes also affect the stability and radioactive properties of an element. Some isotopes are stable and do not undergo radioactive decay, while others are unstable and undergo decay over time, releasing radiation in the process. Isotopes that are radioactive are called radioisotopes, and they can be used in various applications such as medical imaging, cancer treatment, and dating archaeological artifacts.
Key points about isotopes:
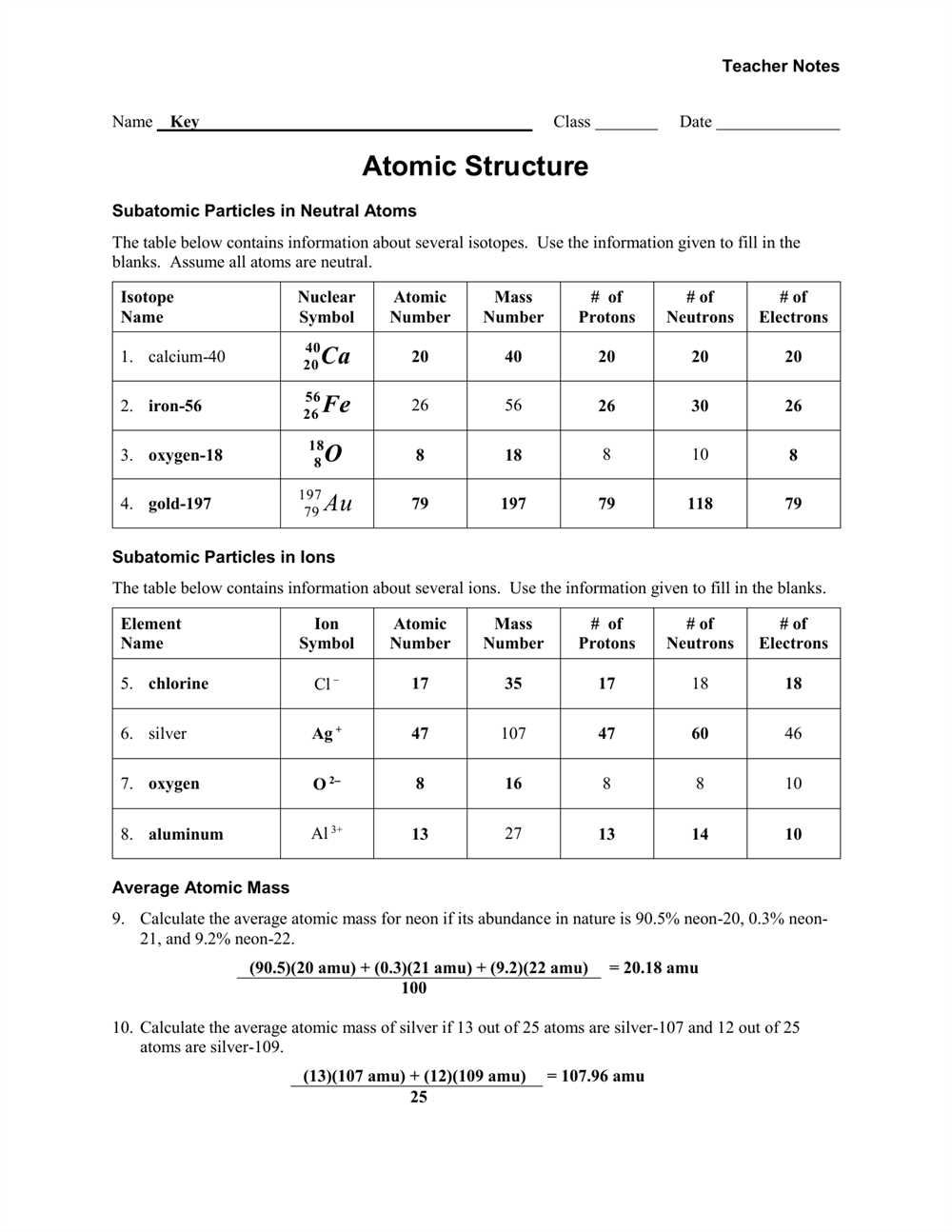
- Isotopes have the same number of protons but different numbers of neutrons.
- Isotopes have different atomic masses.
- The atomic mass of an element is a weighted average of the masses of its isotopes.
- Some isotopes are stable, while others are radioactive.
- Radioisotopes have various uses in different fields.
The Importance of Isotopes in Various Fields
Isotopes play a significant role in a wide range of fields, including chemistry, geology, medicine, environmental science, and archaeology. The unique properties of isotopes make them invaluable in various applications, allowing scientists to understand and analyze crucial aspects of our natural world.
Chemistry: Isotopes are used in chemical reactions to understand reaction mechanisms, determine reaction rates, and study the behavior of atoms and molecules. Isotope labeling is particularly useful in tracing the pathway of chemical reactions, identifying intermediates, and investigating complex biological processes such as protein synthesis.
Geology: Isotopes enable geologists to determine the age of rocks, minerals, and fossils through radioactive dating. This information is essential for understanding Earth’s history, reconstructing past climates, and identifying geological processes. Isotopic analysis also helps identify the origins and transport pathways of water, such as groundwater contamination or the movement of ocean currents.
Medicine: Isotopes find extensive use in medical diagnostics and treatments. Radioactive isotopes, such as technetium-99m, are commonly employed in imaging techniques like positron emission tomography (PET) scans and single-photon emission computed tomography (SPECT) scans. Isotopes also play a crucial role in radiation therapy, where targeted radiation is delivered to cancer cells to destroy them.
Environmental Science: Isotopic analysis helps scientists track the movement and distribution of pollutants and contaminants in ecosystems. By analyzing isotopic signatures, researchers can determine the sources of pollution, the impact of human activities on the environment, and identify long-term trends. This information aids in implementing effective environmental management strategies and policies.
Archaeology: Isotopic analysis aids archaeologists in understanding ancient societies and their cultural practices. By studying the isotopic composition of human remains, scientists can infer dietary habits, migration patterns, and social structures of past civilizations. Isotopes also help determine the origins of artifacts, such as the source of metals used in ancient tools or the geographical origin of pottery.
In conclusion, isotopes play a crucial role in various scientific fields. They provide valuable insights into chemical reactions, geological processes, medical diagnostics and treatment, environmental monitoring, and archaeological studies. The ability to distinguish between different isotopes allows scientists to delve deeper into the intricate workings of our world and make significant contributions to numerous disciplines.
How are isotopes identified?
Isotopes are identified through various methods and techniques. One common method is through mass spectrometry, which involves the measurement of the masses of individual atoms or molecules. By comparing the mass of an atom to the known mass of a specific element, scientists can determine if it is an isotope. Mass spectrometry can also provide information about the relative abundance of different isotopes.
Another method for identifying isotopes is through the use of nuclear magnetic resonance (NMR) spectroscopy. NMR spectroscopy measures the interaction of atomic nuclei with a magnetic field, providing information about their chemical environment and structure. This technique is particularly useful for identifying isotopes in organic compounds.
Isotopes can also be identified through their radioactive decay. Radioactive isotopes, also known as radioisotopes, undergo spontaneous decay, emitting radiation in the process. By measuring the type and amount of radiation emitted, scientists can determine the specific isotope present. This is particularly useful in fields like archaeology and geology, where isotopes can be used to determine the age of artifacts or the composition of rocks and minerals.
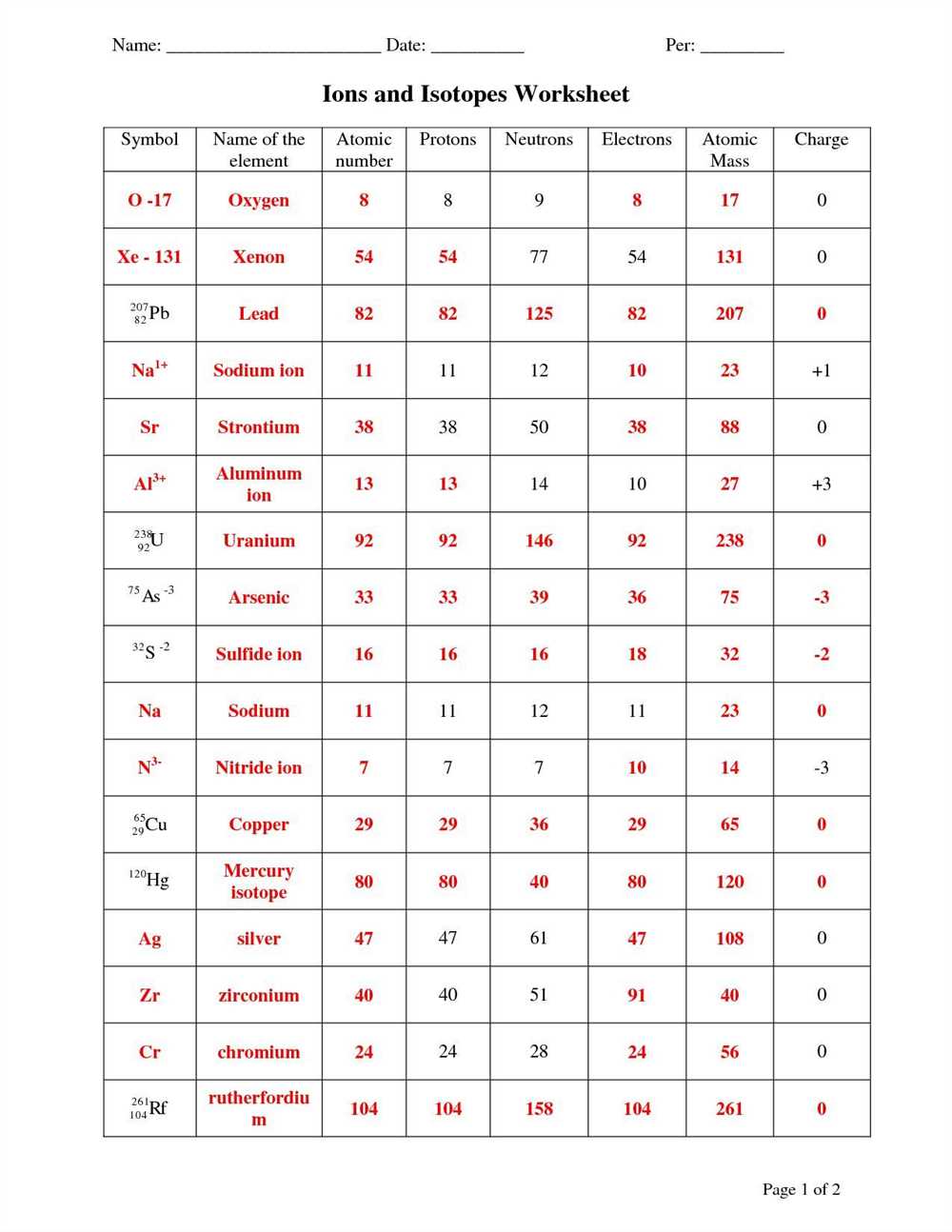
Isotopes, Ions, and Atoms Worksheet: Understanding the Building Blocks of Matter
In the study of chemistry, we often encounter the concepts of isotopes, ions, and atoms. These are fundamental building blocks of matter that help us understand the composition and behavior of different elements.
Isotopes:
Isotopes refer to variations of an element that have the same number of protons but different numbers of neutrons in their atomic nuclei. This results in isotopes having different atomic masses. For example, carbon-12, carbon-13, and carbon-14 are isotopes of carbon. Despite having a different number of neutrons, isotopes of an element have similar chemical properties.
Ions:
Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. When atoms gain electrons, they become negatively charged ions, called anions. Conversely, when atoms lose electrons, they become positively charged ions, called cations. Ions play a crucial role in chemical reactions and the formation of compounds.
Atoms:
Atoms are the basic units of matter and are composed of subatomic particles such as protons, neutrons, and electrons. Protons carry a positive charge, neutrons carry no charge, and electrons carry a negative charge. The number of protons determines the element’s identity, while the combined number of protons and neutrons determines its atomic mass.
Understanding the concepts of isotopes, ions, and atoms is essential in various scientific disciplines, including chemistry, physics, and biology. These concepts help scientists study and manipulate matter, explain chemical reactions, and understand the behavior of elements in the periodic table.
Answers to the Worksheet Questions
In the worksheet on isotopes, ions, and atoms, several questions were given to test your understanding of these concepts. Here are the answers to the questions:
- What are isotopes?
- How do you calculate the average atomic mass of an element?
- What is an ion?
- How do you determine the charge of an ion?
- What is an atom?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They have the same atomic number but different atomic masses.
The average atomic mass of an element is calculated by taking the weighted average of the masses of all its isotopes, where the weights are the relative abundances of each isotope. The formula to calculate the average atomic mass is: (mass of isotope 1 × abundance of isotope 1) + (mass of isotope 2 × abundance of isotope 2) + …
An ion is an atom or a group of atoms that has gained or lost electrons, resulting in a positive or negative charge. Positively charged ions are called cations, and negatively charged ions are called anions.
The charge of an ion depends on the number of electrons gained or lost. If an atom loses electrons, it becomes positively charged, and the charge is equal to the number of electrons lost. If an atom gains electrons, it becomes negatively charged, and the charge is equal to the number of electrons gained.
An atom is the smallest unit of a chemical element that retains the chemical properties of that element. It consists of a nucleus, which contains protons and neutrons, and electrons that orbit the nucleus.
These answers should help clarify the concepts of isotopes, ions, and atoms discussed in the worksheet.