
Understanding electron configurations is essential for understanding the behavior and properties of atoms. In chemistry, electron configurations describe the distribution of electrons in an atom’s orbitals. This distribution determines an atom’s chemical reactivity and its physical properties.
Electron configurations are represented by a series of numbers and letters that correspond to different energy levels, sublevels, and orbitals. The numbers indicate the principal energy levels, while the letters represent the sublevels (s, p, d, f). Each sublevel can hold a specific number of electrons, with s holding 2 electrons, p holding 6 electrons, d holding 10 electrons, and f holding 14 electrons.
The electron configuration of an atom can be determined using the periodic table and the knowledge of electron filling rules. Aufbau principle states that electrons fill the lowest energy level orbitals first before moving to higher energy levels. Hund’s rule states that within a sublevel, electrons will fill each orbital singly before pairing up. Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins. These rules allow us to predict the electron configurations of atoms and understand their stability.
Electron configurations answer key provides a concise and organized summary of the electron configurations for all the elements in the periodic table. It serves as a valuable reference tool for students and researchers in the field of chemistry. By understanding and interpreting the electron configurations, scientists can gain insights into the behavior and properties of atoms, which can be applied in various fields including materials science, pharmacy, and environmental studies.
What are Electron Configurations?
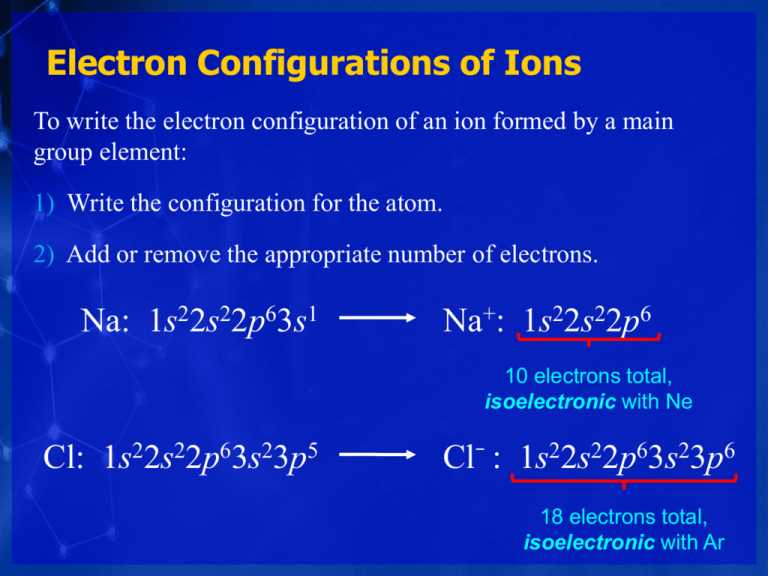
An electron configuration is a representation of the arrangement of electrons in an atom or molecule. It describes the energy levels, or shells, that the electrons occupy, as well as the number of electrons in each shell. This configuration is crucial in understanding the chemical properties and behavior of an element.
Electrons in an atom are located in specific energy levels, which are further divided into sublevels. Each sublevel can hold a certain maximum number of electrons. The electron configuration of an atom follows a specific pattern, known as the Aufbau principle, which states that electrons fill the lowest available energy levels before occupying higher energy levels.
The electron configuration is typically represented using a shorthand notation, where the energy levels are labeled with numbers and the sublevels are represented by letters: s, p, d, and f. The number of electrons in each sublevel is indicated as a superscript after the letter. For example, the electron configuration of oxygen (O) is 1s2 2s2 2p4, which means that it has 2 electrons in the 1s sublevel, 2 electrons in the 2s sublevel, and 4 electrons in the 2p sublevel.
The electron configuration of an element determines its chemical properties and reactivity. It helps predict how an element will interact with other elements and form compounds. The electron configuration also provides insights into the stability of an atom, as well as its ability to gain or lose electrons to form ions.
- The Aufbau principle guides the filling of electron energy levels.
- Electron configuration is represented using a shorthand notation.
- It determines the chemical properties and reactivity of an element.
Understanding the Periodic Table
The periodic table is a comprehensive tool used by scientists to organize and understand the elements. It can be thought of as a map that shows the relationships and patterns between different elements. The layout of the periodic table is based on the atomic number and electron configuration of each element.
The atomic number represents the number of protons found in the nucleus of an atom, while electron configuration refers to the arrangement of electrons within an atom’s energy levels or orbitals. By understanding the electron configuration, scientists can determine an element’s chemical reactivity and its position in the periodic table.
The periodic table is divided into several sections, including periods and groups. Periods are horizontal rows, while groups are vertical columns. Each element within a period has its own unique electron configuration, which follows a specific pattern. For example, elements in group 1, also known as the alkali metals, have one electron in their outermost energy level, while elements in group 18, the noble gases, have full outermost energy levels.
The periodic table also provides valuable information about an element’s physical properties, such as its atomic mass, melting point, and boiling point. This information is essential for scientists in predicting and understanding the behavior of different elements in chemical reactions and other processes.
In conclusion, the periodic table is a crucial tool for scientists to organize, understand, and predict the behavior of elements. By studying the electron configuration and other properties of elements, scientists can gain valuable insights into the periodic trends and patterns that exist within the table.
The Aufbau Principle
The Aufbau Principle is a fundamental concept in chemistry that governs the arrangement of electrons in an atom. According to this principle, electrons fill the available atomic orbitals in order of increasing energy level. This means that lower energy levels must be filled before higher energy levels.
The Aufbau Principle is based on the idea that electrons occupy specific energy levels within an atom. These energy levels are often referred to as electron shells or electron orbitals. Each energy level can hold a specific number of electrons, with the first level closest to the nucleus holding a maximum of 2 electrons, the second level holding a maximum of 8 electrons, and so on.
When filling electron orbitals, electrons follow a specific pattern. The principle states that electrons will first fill the lowest energy level available before moving on to higher levels. This means that the 1s orbital will be filled before the 2s orbital, and so on. Within each energy level, electrons also follow a specific pattern. For example, the 2p orbital will be filled after the 2s orbital.
Using the Aufbau Principle, scientists can determine the electron configuration of an atom. This configuration represents the distribution of electrons in the various energy levels and orbitals. For example, the electron configuration of nitrogen (N) is 1s2 2s2 2p3, which means that nitrogen has 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 3 electrons in the 2p orbital.
Hund’s Rule
The Hund’s Rule is a fundamental principle in quantum mechanics that governs the way electrons occupy atomic orbitals. It states that when electrons occupy orbitals of the same energy level, they will first fill each orbital with a single electron before pairing up. This rule ensures that the electronic configuration of an atom is in its lowest energy state, known as the ground state.
According to Hund’s Rule, electrons are considered to have the same energy level if they have the same principal quantum number (n) and are in different subshells. For example, in the carbon atom (atomic number 6), the 2p subshell contains three orbitals (px, py, and pz) that can hold a total of six electrons. When filling these orbitals, each orbital will be populated with one electron before any orbital begins to pair up.
The Hund’s Rule can be represented using the “up and down arrow” notation, where each arrow represents an electron. For example, for the carbon atom, the electronic configuration can be represented as 1s2 2s2 2p2, with the 2px, 2py, and 2pz orbitals each having one electron with opposite spins.
In summary, Hund’s Rule dictates that electrons will occupy individual orbitals before pairing up, ensuring that the electronic configuration of an atom is in its lowest energy state. This principle plays a crucial role in understanding the arrangement of electrons in atoms and the chemical properties of elements.
Pauli Exclusion Principle
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics that states that no two electrons in an atom can have the same set of quantum numbers. This means that electrons must occupy different energy levels and have opposite spins in order to exist in the same orbital.
The principle was first formulated by Austrian physicist Wolfgang Pauli in 1925. It is based on the concept of electron spin, which is an intrinsic property of electrons that determines their orientation in space. Each electron has a unique set of quantum numbers, including its principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number.
According to the Pauli Exclusion Principle, no two electrons in an atom can have the same four quantum numbers. This means that each orbital can accommodate a maximum of two electrons, with opposite spins. In other words, electrons fill up orbitals in pairs, with one spinning in one direction and the other spinning in the opposite direction.
This principle has important consequences for the structure and behavior of atoms. It explains why elements have distinct electron configurations and why certain elements have higher or lower ionization energies. It also plays a crucial role in determining the shape and stability of molecules.
In summary, the Pauli Exclusion Principle dictates that electrons in an atom must have unique sets of quantum numbers, leading to distinct electron configurations and behavior. This principle is fundamental to our understanding of quantum mechanics and has wide-ranging implications in chemistry and physics.
Orbital Diagrams and Electron Configurations
In chemistry and physics, orbital diagrams and electron configurations are used to represent the arrangement of electrons in an atom. These representations help us understand the behavior and properties of elements.
An orbital diagram visually depicts the orbitals of an atom and how they are occupied by electrons. Each orbital is represented by a box, and the arrows inside the boxes represent the electrons. The arrows point up or down to indicate the spin of the electron. The orbital diagram follows Aufbau’s principle, which states that electrons fill the lowest energy orbitals first.
The electron configuration is a shorthand notation that represents the distribution of electrons in the orbitals. It is written using the symbol for the atomic element followed by a series of numbers and letters. The numbers represent the principal energy level and the letters represent the sublevel or orbital type.
For example, the electron configuration of oxygen is 1s^2 2s^2 2p^4. This means that oxygen has 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 4 electrons in the 2p orbital. The superscript numbers indicate the number of electrons in each orbital.
Understanding orbital diagrams and electron configurations is essential in determining the chemical reactivity and bonding behavior of elements. It allows scientists to predict the behavior of elements and their compounds, as well as explain periodic trends and properties.
Writing Electron Configurations

In chemistry, an electron configuration is a description of how electrons are arranged in an atom or a molecule. It is represented by a series of numbers and letters that indicate the energy levels, sublevels, and number of electrons in each. The electron configuration provides important information about an element’s properties and behavior.
When writing the electron configuration for an atom, it is important to follow a specific format. The format starts with the energy level, followed by the sublevel, and then the number of electrons in that sublevel. The energy levels are represented by numbers: 1, 2, 3, and so on. The sublevels are represented by letters: s, p, d, and f. The number of electrons in each sublevel is indicated by superscripts. For example, the electron configuration for hydrogen would be written as 1s1.
The periodic table can be used as a tool to determine the electron configuration of an element. Each element corresponds to a specific position on the table, and its electron configuration can be derived from its position. For example, helium is located in the first row of the table and has an atomic number of 2. Its electron configuration is 1s2, indicating that it has two electrons in the first energy level, both in the s sublevel.
Electron configurations can also be written in shorthand notation using noble gas notation. Noble gases are the elements in group 18 of the periodic table, and they have completely filled energy levels. By using noble gas notation, the electron configuration of an element can be shortened by representing the noble gas that comes before it and indicating only the additional electrons. For example, the electron configuration of oxygen can be written as [He] 2s2 2p4, where [He] represents the electron configuration of helium.
Summary:
- Electron configurations describe how electrons are arranged in atoms or molecules.
- They provide important information about an element’s properties and behavior.
- The format for writing electron configurations includes energy levels, sublevels, and the number of electrons in each sublevel.
- The periodic table can be used to determine the electron configuration of an element.
- Shorthand notation using noble gas notation can be used to represent electron configurations more efficiently.
Electron Configurations Answer Key
The electron configuration of an atom describes how electrons are distributed in its atomic orbitals. It is represented by a series of numbers and letters that indicate the energy levels and types of orbitals occupied by electrons. Understanding electron configurations is essential in predicting an atom’s reactivity and chemical behavior.
In this answer key, we will provide the electron configurations for various elements. Each element has a unique electron configuration that follows certain rules. The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy levels. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins. Hund’s rule states that electron pairing in orbitals of the same energy level occurs only after each orbital has one electron.
Here are a few examples of electron configurations:
- Hydrogen (H): 1s1
- Carbon (C): 1s2 2s2 2p2
- Oxygen (O): 1s2 2s2 2p4
- Iron (Fe): 1s2 2s2 2p6 3s2 3p6 4s2 3d6
The electron configuration determines an atom’s chemical properties and how it can form bonds with other atoms. It helps in understanding the periodic trends such as ionization energy, electron affinity, and atomic radius. By knowing the electron configuration, scientists can predict an element’s behavior in chemical reactions and its placement in the periodic table.