Chemical bonding is a fundamental concept in chemistry, forming the basis for understanding how atoms come together to create molecules and compounds. This concept helps explain why certain elements are reactive and others are not, and how different properties, such as melting and boiling points, are determined.
The study of chemical bonding focuses on the interactions between atoms, which can be either covalent or ionic in nature. Covalent bonding involves the sharing of electrons between atoms, while ionic bonding occurs when electrons are transferred from one atom to another. Understanding these different types of bonding is crucial for understanding the behavior and properties of different materials.
This article will provide a comprehensive review of the key concepts related to chemical bonding, including Lewis structures, electronegativity, and molecular shapes. By the end, you will have a solid understanding of the basic principles of chemical bonding and be able to apply them to analyze and predict the behavior of different molecules and compounds.
Whether you are a student studying chemistry for the first time or someone looking to refresh their knowledge, this article will serve as a valuable resource to help you grasp the fundamental concepts of chemical bonding.
Chemical Bonding: An Essential Concept in Chemistry
Chemical bonding is the phenomenon that allows atoms to combine and form chemical compounds. It is a fundamental concept in chemistry because it explains how atoms interact and why certain elements bond together to create stable structures. Without chemical bonding, the world as we know it would not exist, as most substances are formed through the interaction of different atoms.
Atoms are the building blocks of matter, and they consist of a nucleus containing protons and neutrons, surrounded by electrons in specific energy levels. Chemical bonding occurs when electrons are shared or transferred between atoms. This interaction allows atoms to achieve a more stable electron configuration, following the octet rule, which states that atoms tend to gain, lose, or share electrons to attain a full outer electron shell with eight valence electrons.
There are three main types of chemical bonding: ionic bonding, covalent bonding, and metallic bonding. In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions), which are attracted to each other due to their opposite charges. Covalent bonding, on the other hand, involves the sharing of electron pairs between atoms, forming molecules. Metallic bonding occurs in metals, where electrons are delocalized and are free to move throughout the structure.
Chemical bonding plays a crucial role in determining the properties of substances. Different bonding types result in different properties such as melting point, boiling point, solubility, conductivity, and hardness. Understanding chemical bonding allows chemists to predict and explain the behavior of substances and design new materials with specific properties.
Why is Chemical Bonding Important?

Chemical bonding is a fundamental concept in chemistry that plays a crucial role in understanding the structure, properties, and behavior of matter. Understanding chemical bonding allows scientists to predict how different atoms will interact and combine to form new substances.
One key reason why chemical bonding is important is that it determines the physical and chemical properties of compounds and elements. For example, the type and strength of chemical bonding in a substance influence its melting and boiling points, conductivity, solubility, and reactivity. By understanding the nature of chemical bonding, scientists can design materials with specific properties for various applications.
Chemical bonding is also essential in explaining and predicting the behavior of substances in chemical reactions. When atoms bond together, they share or transfer electrons to achieve a more stable electron configuration. This transfer or sharing of electrons determines the formation of chemical bonds and the overall structure of molecules. By understanding the concept of bonding, scientists can predict how different substances will react with each other and form new compounds. This knowledge is crucial in fields such as pharmaceuticals, materials science, and environmental studies.
In summary, chemical bonding is crucial in understanding the structure, properties, and behavior of matter. It allows scientists to predict and manipulate the properties of substances, as well as explain and predict their behavior in chemical reactions. Without a thorough understanding of chemical bonding, many advancements in chemistry and related fields would not be possible.
Types of Chemical Bonds
Chemical bonds are the attractive forces that hold atoms together in compounds. There are three main types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds. Each type of bond involves the sharing or transfer of electrons between atoms.
Ionic bonds occur when electrons are transferred from one atom to another. In this type of bond, one atom becomes positively charged (cation) and the other becomes negatively charged (anion). The attraction between the opposite charges holds the atoms together. Ionic bonds typically occur between metals and nonmetals.
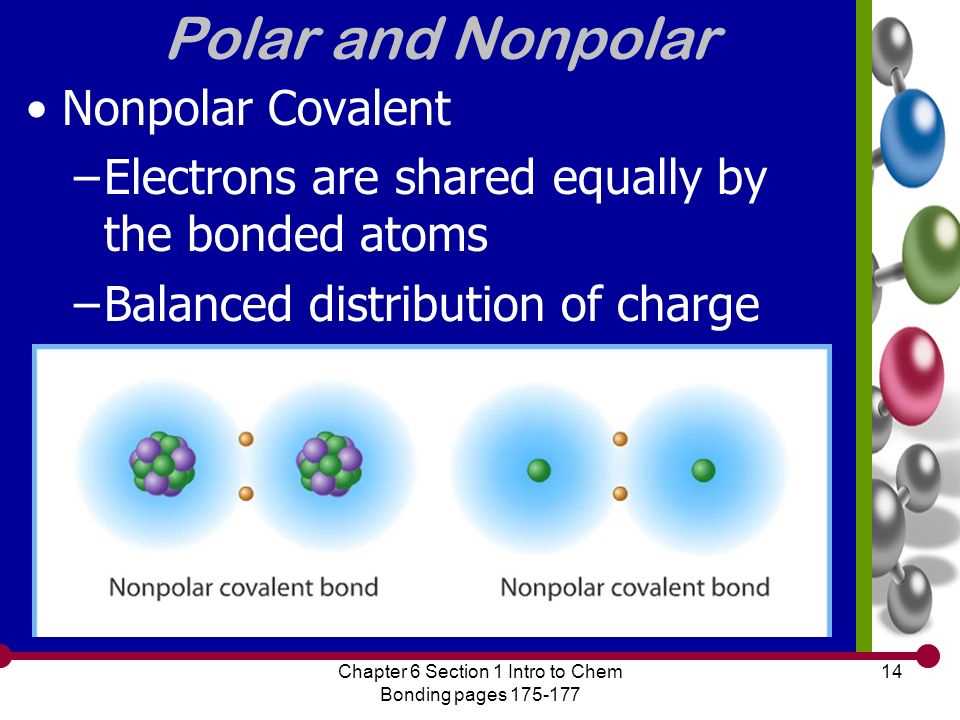
Covalent bonds occur when atoms share electrons. In this type of bond, the atoms achieve a stable electron configuration by sharing electrons with each other. Covalent bonds usually occur between nonmetals. There are two types of covalent bonds: polar covalent bonds and nonpolar covalent bonds. Polar covalent bonds occur when there is an unequal sharing of electrons, resulting in a slight positive and negative charge on different parts of the molecule. Nonpolar covalent bonds occur when electrons are shared equally between atoms.
Metallic bonds occur between metal atoms. In this type of bond, the valence electrons of metal atoms are delocalized and free to move throughout the metal lattice. This accounts for the high electrical conductivity and malleability of metals. Metallic bonds are strong and are responsible for the characteristic properties of metals.
In summary, the three main types of chemical bonds are ionic, covalent, and metallic. These bonds play a crucial role in determining the properties and behaviors of different substances.
Ionic Bonds
Ionic bonds are formed when there is a complete transfer of electrons between two atoms. This transfer occurs between a metal and a nonmetal, resulting in the formation of ions. Ions are charged particles that are formed when an atom gains or loses electrons. In an ionic bond, the metal atom loses one or more electrons to become a positively charged cation, while the nonmetal atom gains one or more electrons to become a negatively charged anion.
The formation of ionic bonds is driven by the attraction between the positive and negative charges of the ions. The positively charged cations are attracted to the negatively charged anions, creating a strong bond between the two atoms. This attraction is known as electrostatic attraction. Ionic bonds are typically found in compounds such as salts, which consist of a lattice structure of alternating cations and anions.
When an ionic bond forms, there is a transfer of electrons from one atom to another. This transfer results in the formation of a stable compound. The combining ratio of the elements in an ionic compound is determined by the charges on the ions. The charges on the ions are determined by the number of electrons gained or lost. For example, if a metal atom loses one electron, it becomes a cation with a positive charge of +1. If a nonmetal atom gains one electron, it becomes an anion with a negative charge of -1. The resulting compound will then have a combining ratio of 1:1.
Overall, ionic bonds are characterized by the transfer of electrons between atoms, resulting in the formation of oppositely charged ions. This electrostatic attraction between the ions leads to the formation of stable compounds with a specific combining ratio. The presence of ionic bonds is responsible for the unique properties and behaviors exhibited by ionic compounds.
Covalent Bonds
Covalent bonds are formed when two atoms share electrons in order to achieve a stable electron configuration. This type of bonding typically occurs between nonmetals or between a nonmetal and a metalloid. The shared electrons move in the space between the two atoms, creating a bond that holds the atoms together. Covalent bonds are typically stronger than ionic bonds, but weaker than metallic bonds.
When two atoms form a covalent bond, they each contribute one or more electrons to a shared electron pair. The electrons in the shared pair are attracted to the positive nuclei of both atoms, creating a stable bond. The number of electrons shared between atoms can vary, ranging from one electron pair (single bond) to two electron pairs (double bond) or three electron pairs (triple bond).
Covalent bonds can also form between atoms of the same element, resulting in the formation of diatomic molecules. For example, the covalent bond between two oxygen atoms forms an oxygen molecule (O2), while the covalent bond between two nitrogen atoms forms a nitrogen molecule (N2). These diatomic molecules are stable because the shared electron pairs provide both atoms with a full outer electron shell.
Overall, covalent bonds play a crucial role in the formation of molecules and compounds. They determine the physical and chemical properties of substances and are responsible for the unique characteristics of different materials. Understanding covalent bonds is essential in chemistry, as it allows us to explain and predict the behavior of various substances.
Metallic Bonds
A metallic bond is a type of chemical bonding that occurs between atoms in a metal. It is characterized by the sharing of electrons between a lattice of positively charged metal ions and a sea of delocalized electrons. This sharing of electrons allows metals to conduct electricity and heat, as well as exhibit high melting and boiling points.
In a metallic bond, the valence electrons of the metal atoms are free to move throughout the lattice, creating a cloud of electrons that surrounds the positively charged metal ions. This electron cloud is responsible for the unique properties of metals, such as their malleability and ductility. The delocalized nature of the electrons also allows metals to form alloys by mixing different metal atoms together.
One of the key factors that determines the strength of a metallic bond is the number of valence electrons available for bonding. Metals with more valence electrons tend to have stronger metallic bonds, as there are more electrons available to form the electron cloud. Additionally, the size of the metal ions can also affect the strength of the metallic bond, as smaller ions can pack more closely together, leading to stronger bonding.
Overall, metallic bonds are essential for the properties and behaviors of metals. They allow metals to be good conductors of electricity and heat, have high melting and boiling points, and possess unique physical properties. Understanding metallic bonding is crucial for comprehending the behavior of metal materials in various applications, from electrical wiring to structural engineering.
Properties of Chemical Bonds
Chemical bonds are the forces that hold atoms together in a molecule or compound. These bonds are formed when electrons are shared, transferred, or attracted between atoms. The properties of chemical bonds play a crucial role in determining the physical and chemical properties of substances.
1. Bond Length: The bond length is the distance between the two nuclei of the bonded atoms. It is influenced by factors such as the size and electronegativity of the atoms involved in the bond. Generally, shorter bond lengths indicate stronger bonds.
2. Bond Energy: Bond energy, also known as bond strength, is the energy required to break a chemical bond and separate the atoms. It is a measure of the stability of the bond. Higher bond energy indicates a stronger bond.
3. Polarity: Some chemical bonds result in the formation of polar molecules, where there is an uneven distribution of electron density. This occurs when there is a significant difference in electronegativity between the bonded atoms. Polar bonds have distinct positive and negative poles.
4. Molecular Geometry: The arrangement of atoms in a molecule is determined by the type and number of chemical bonds. This arrangement, also known as molecular geometry, affects the overall shape and properties of the molecule.
5. Solubility and Melting/Boiling Points: The strength and nature of chemical bonds influence the solubility of substances in different solvents and their melting and boiling points. Substances with stronger bonds tend to have higher melting and boiling points and lower solubility.
Understanding the properties of chemical bonds is essential in various fields, such as chemistry, materials science, biochemistry, and pharmacology. It allows scientists to predict the behavior of substances and develop new materials with desired properties.
Strength of Chemical Bonds
The strength of a chemical bond is determined by the forces holding the atoms together. This strength can vary depending on the types of atoms involved in the bond and the arrangement of electrons within the bond. Understanding the strength of chemical bonds is crucial in predicting and explaining the properties and behaviors of substances, as well as in designing and synthesizing new materials with desired properties.
One way to measure the strength of a chemical bond is through bond energy, which is the amount of energy required to break a bond and separate the atoms. Bond energy is typically expressed in kilojoules per mole (kJ/mol) and can be found by experimental methods or calculated using theoretical models. Higher bond energies indicate stronger bonds, and substances with stronger bonds are generally more stable and less reactive.
There are several factors that influence the strength of chemical bonds. The first is the type of bond formed between atoms. Ionic bonds, which occur between ions of opposite charges, tend to have higher bond energies than covalent bonds, which involve the sharing of electrons between atoms. Metallic bonds, which occur in metals, also have high bond energies due to the delocalization of electrons throughout the structure.
The arrangement and number of electrons within a bond also affect its strength. Bonds with multiple bonding electrons, such as double or triple bonds, are generally stronger than bonds with only single bonding electrons. Additionally, the arrangement of electrons within a bond can give rise to different types of bonding, such as polar or nonpolar covalent bonds, which have different strengths.
In summary, the strength of chemical bonds is determined by the forces holding the atoms together, and this strength can be measured using bond energy. Factors such as the type of bond and the arrangement of electrons within the bond can influence its strength. Understanding the strength of chemical bonds is essential in various scientific and technological applications, from designing new materials to understanding the behavior of substances.