In order to understand the behavior of atoms and their chemical properties, it is essential to have a solid understanding of electron configuration. The periodic table provides a systematic way of organizing elements based on their atomic structure and electron arrangement.
This review packet will serve as a comprehensive guide to help you navigate through the intricacies of electron configurations. By utilizing the answer key provided, you will be able to consolidate your knowledge and reinforce your understanding of how electrons are distributed in different energy levels and orbitals.
The answer key will not only provide you with the correct electron configurations for each element, but it will also explain the logic behind the arrangement. You will learn about the principles of electron filling, the importance of electron spin, and the rules governing the placement of electrons in various atomic orbitals.
By using this review packet and its answer key, you will strengthen your grasp of electron configuration and gain a deeper appreciation for the organization and patterns present in the periodic table. Whether you are a student studying chemistry or a professional looking to refresh your knowledge, this resource will be invaluable in solidifying your understanding of electron arrangement and its significance in chemistry.
Electrons Periodic Table Review Packet Answer Key
In this answer key, we will review the concepts and information related to electrons in the periodic table. It is important to understand the behavior and properties of electrons as they play a crucial role in determining the chemical properties of elements. Understanding how electrons are organized in the periodic table is fundamental for understanding the periodic trends and bonding between atoms.
1. Electron Configuration: The arrangement of electrons in an atom is represented by its electron configuration. The electron configuration is determined by the principles of the Aufbau principle, Pauli exclusion principle, and Hund’s rule. The electron configuration is typically written using the following notation: 1s^2, 2s^2, 2p^6, and so on, where the numbers and letters represent the energy levels and sublevels.
2. Valence Electrons: Valence electrons are the electrons in the outermost energy level of an atom. These electrons are involved in chemical bonding and determine the reactivity and chemical properties of elements. In the periodic table, the number of valence electrons increases from left to right in a period and from top to bottom in a group.
- To find the number of valence electrons for an atom, look at its group number (column) in the periodic table.
- The main group elements have valence electrons equal to their group number (e.g., Group 1 elements have 1 valence electron, Group 2 elements have 2 valence electrons).
- Transition metals have valence electrons distributed in multiple energy levels.
3. Electron Orbitals: Electron orbitals are the regions in an atom where electrons are most likely to be found. Each energy level contains one or more orbitals, and each orbital can hold a maximum of two electrons with opposite spins. The s, p, d, and f orbitals have different shapes and orientations within an energy level.
| Energy Level | Sublevel | Max. Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s, 2p | 8 |
| 3 | 3s, 3p | 8 |
| 4 | 4s, 3d, 4p | 18 |
| 5 | 5s, 4d, 5p | 18 |
| 6 | 6s, 4f, 5d, 6p | 32 |
| 7 | 7s, 5f, 6d, 7p | 32 |
By understanding the electron configuration, valence electrons, and electron orbitals, we can gain a deeper understanding of the periodic table and how chemical reactions occur. This knowledge is essential for studying chemistry and exploring the properties and behaviors of different elements.
Structure of the Atom and Electron Configuration
The atom is the basic unit of matter and is composed of three main subatomic particles: protons, neutrons, and electrons. Protons have a positive charge and are located in the nucleus of the atom, along with neutrons, which have no charge. Electrons, on the other hand, have a negative charge and orbit the nucleus in specific energy levels.
The electron configuration of an atom refers to the arrangement of electrons in its energy levels. Each energy level can only hold a certain number of electrons, with the first energy level being able to hold a maximum of two electrons, and subsequent levels being able to hold up to eight electrons. The arrangement of electrons in the energy levels follows a set of rules known as the Aufbau principle, the Pauli exclusion principle, and Hund’s rule.
The Aufbau principle states that electrons fill the lowest energy levels first before moving to higher energy levels. The Pauli exclusion principle states that each orbital within an energy level can only hold a maximum of two electrons, and these electrons must have opposite spins. Hund’s rule states that when filling orbitals of equal energy, electrons will occupy each orbital singly before pairing up.
The electron configuration of an atom can be represented using a series of numbers and letters. The numbers represent the energy levels, while the letters represent the specific sublevels within each energy level. For example, the electron configuration of oxygen is 1s² 2s² 2p⁴, which means there are two electrons in the first energy level (1s), two electrons in the second energy level (2s), and four electrons in the second energy level (2p).
Understanding the structure of the atom and electron configuration is important in understanding the chemical properties and behavior of elements. It helps determine how elements interact with each other to form compounds, and it allows scientists to predict the behavior and properties of unknown elements based on their position in the periodic table.
Understanding Electron Orbitals and Energy Levels
The concept of electron orbitals and energy levels is fundamental to understanding the behavior and arrangement of electrons in an atom. Orbitals are regions around the nucleus where electrons are most likely to be found. They can accommodate a maximum of two electrons with opposite spins. Energy levels, on the other hand, refer to the different energy states available to electrons within an atom.
Electron orbitals are represented by different shapes and are labeled with a combination of letters and numbers. The principal quantum numbers (n) classify the main energy levels, with higher values of n corresponding to higher energy levels. The secondary quantum number (l) distinguishes the different sublevels within each energy level. The magnetic quantum number (ml) further specifies the orientation of the orbital within each sublevel.
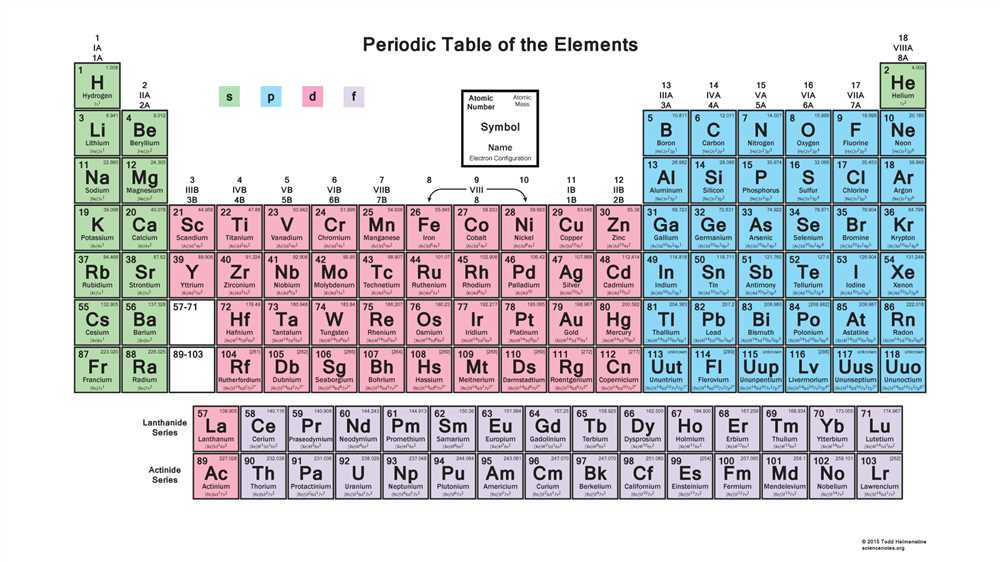
There are four types of orbitals: s, p, d, and f. The s orbitals are spherical in shape and exist within all energy levels. The p orbitals are dumbbell-shaped and exist in the second and higher energy levels. The d and f orbitals have more complex shapes and exist in the third and higher energy levels. Each orbital can hold a maximum number of electrons depending on its shape and orientation.
The arrangement of electrons in an atom follows the aufbau principle, which states that electrons occupy the lowest energy level available before moving to higher energy levels. The electrons are distributed among the different orbitals in a specific order based on their energy. This organization is represented by the electron configuration of an element, which shows the number and arrangement of electrons in each energy level and orbital.
Understanding electron orbitals and energy levels is crucial for predicting chemical behavior and explaining the properties of elements. It allows us to understand why certain elements have similar characteristics and why others exhibit different behaviors. The periodic table is a valuable tool that organizes and displays the electron configurations of elements, providing insights into their behavior and reactivity.
Electron Configurations and the Periodic Table
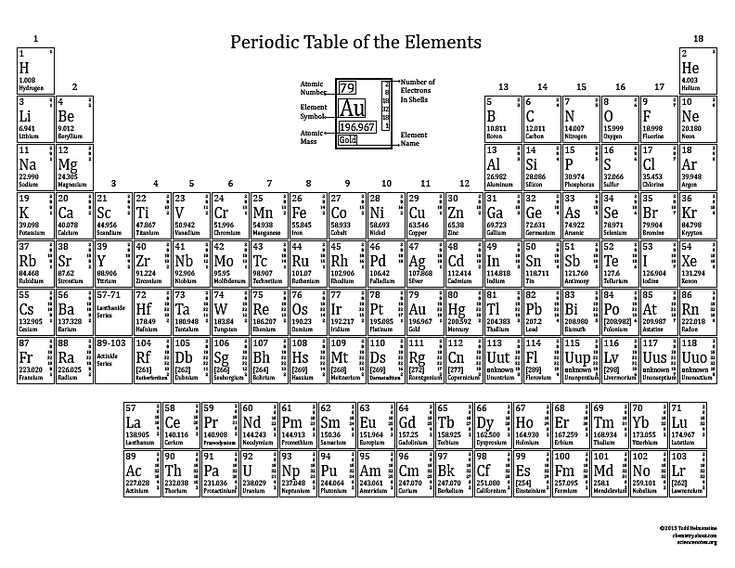
The electron configuration of an atom is a representation of how the electrons are arranged in its energy levels or shells. It provides important information about an atom’s chemical behavior and its position in the periodic table. The periodic table is an organized arrangement of elements based on their atomic number and electron configuration.
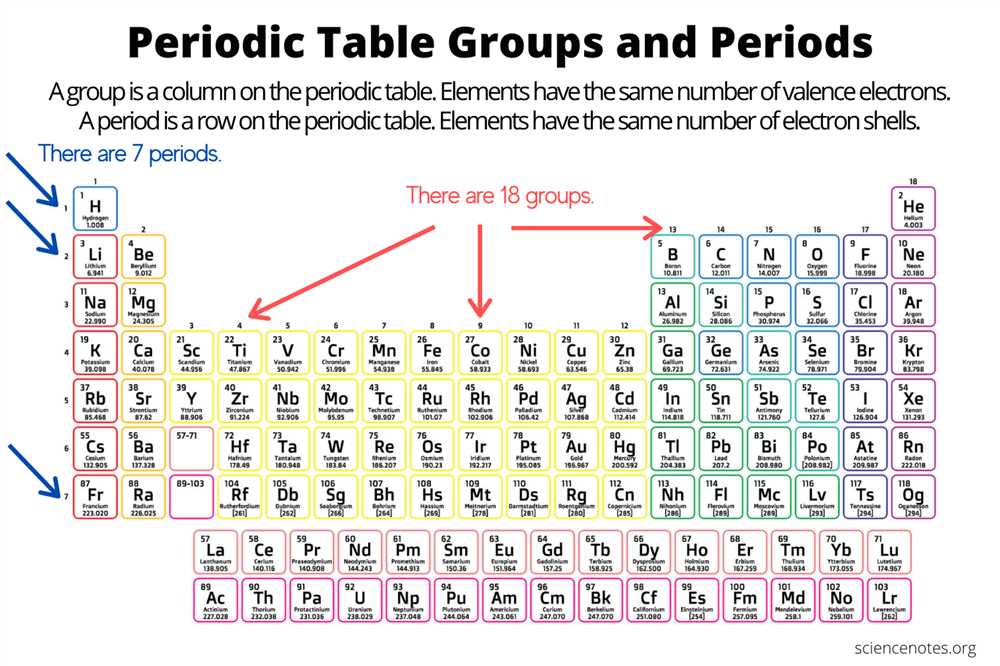
In the periodic table, elements are arranged in rows called periods and columns called groups or families. The elements within a group have similar properties because they have the same number of valence electrons, which are the electrons in the outermost energy level. The number of valence electrons determines an element’s chemical reactivity and its ability to form bonds with other elements.
The periodic table also follows a pattern in the filling of electron orbitals. The first energy level can hold a maximum of 2 electrons, the second energy level can hold a maximum of 8 electrons, and so on. The filling of electron orbitals follows the Aufbau principle, which states that electrons occupy the lowest available energy level first, before filling higher energy levels.
Understanding electron configurations and the periodic table allows us to predict the chemical behavior of elements. Elements in the same group have similar electron configurations and tend to have similar properties. For example, the elements in group 1, known as the alkali metals, all have one valence electron and are highly reactive. On the other hand, elements in group 18, known as the noble gases, have full valence electron shells and are unreactive.
Summary:
- The electron configuration of an atom represents the arrangement of electrons in its energy levels or shells.
- The periodic table is an organized arrangement of elements based on their atomic number and electron configuration.
- Elements within a group have similar properties because they have the same number of valence electrons.
- The filling of electron orbitals follows the Aufbau principle, with electrons occupying the lowest available energy level first.
- Understanding electron configurations and the periodic table allows us to predict the chemical behavior of elements.
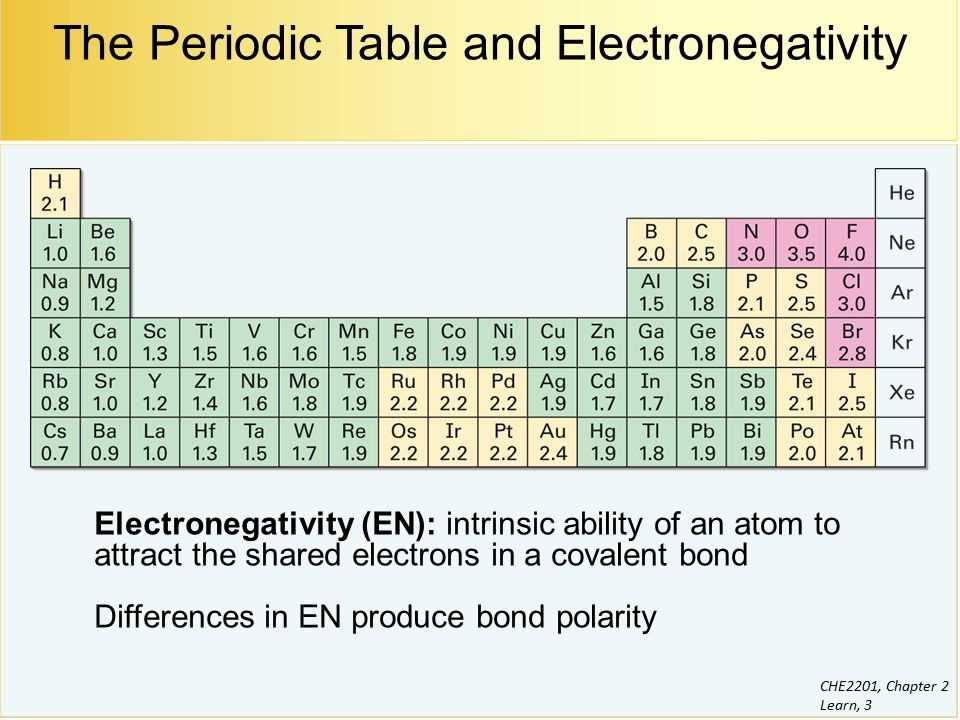
Electron Affinity and Electronegativity
Electron affinity and electronegativity are two important concepts in chemistry that help us understand the behavior of atoms and molecules. Electron affinity refers to the energy change that occurs when an atom gains an electron, while electronegativity is a measure of an atom’s ability to attract electrons towards itself in a chemical bond.
Electron affinity is typically measured in electron volts (eV) and can be either positive or negative. A positive electron affinity indicates that energy is released when an atom gains an electron, while a negative electron affinity suggests that energy must be supplied for an atom to accept an additional electron. Generally, elements on the right side of the periodic table have higher values of electron affinity, as they have a greater tendency to gain electrons and become more stable.
The concept of electronegativity, on the other hand, is particularly useful in understanding how atoms share electrons in a chemical bond. Electronegativity values are assigned to each element on a scale, with fluorine having the highest value of 4.0. The difference in electronegativity between two atoms determines the type of bonding that occurs, such as nonpolar covalent, polar covalent, or ionic. Elements with higher electronegativity values have a stronger pull on shared electrons, resulting in a more polar bond.
- Electron affinity measures the energy change when an atom gains an electron.
- Electronegativity describes an atom’s ability to attract electrons in a chemical bond.
- Elements on the right side of the periodic table typically have higher electron affinity values.
- Electronegativity values determine the type of bonding that occurs between atoms.
By understanding electron affinity and electronegativity, chemists can predict and explain the behavior of atoms and molecules in various chemical processes. These concepts provide valuable insights into the reactivity and bonding patterns of elements, allowing scientists to design and optimize new compounds for various applications.
Trends in Electron Configuration and Reactivity
The electron configuration of an atom refers to the arrangement of electrons in its energy levels or shells. The arrangement of electrons determines the reactivity of an atom, as it dictates how easily the atom can gain or lose electrons. Understanding the trends in electron configuration can provide valuable insights into the chemical behavior of elements.
One important trend in electron configuration is the periodicity of electron shells. As you move across a period in the periodic table, the number of shells increases by one. This means that elements in the same period will have different electron configurations, which can influence their reactivity. For example, elements in the same period as oxygen will have similar electron configurations, making them more likely to form similar compounds.
The periodic table also exhibits trends in electron configuration within each group or column. Elements in the same group have similar outer electron configurations, which contribute to their similar chemical properties. For example, elements in Group 1, such as lithium and sodium, have a single electron in their outermost shell. This makes them highly reactive and likely to lose that outer electron in chemical reactions.
Additionally, the size of the atom can affect its electron configuration and reactivity. As you move down a group on the periodic table, the size of the atoms increases due to the addition of extra energy levels. This can influence the ease with which electrons can be gained or lost, as larger atoms have a greater distance between the outermost electrons and the positively charged nucleus.
In summary, understanding the trends in electron configuration and reactivity can help predict the behavior of elements and their ability to form compounds. The periodicity of electron shells, the similarity of outer electron configurations within groups, and the size of atoms all play a role in determining these trends.