Chemical bonding is a fundamental concept in chemistry that helps us understand how atoms come together to form molecules and compounds. One type of chemical bonding is called ionic bonding, which involves the transfer of electrons between atoms. Understanding the principles of ionic bonding is crucial for unlocking the puzzle of chemical reactions and explaining the properties of various substances.
In the Ionic Bonding Puzzle Lab, students are presented with a hands-on activity to explore and analyze ionic bonding in a fun and interactive way. The lab provides students with a set of puzzle pieces, representing atoms with incomplete electron configurations. By rearranging the puzzle pieces and matching atoms with their corresponding electron configurations, students can visualize and comprehend the process of ionic bonding.
The answer key for the Ionic Bonding Puzzle Lab is an invaluable resource that provides students with the correct combinations of puzzle pieces and electron configurations. It serves as a guide for students to check their solutions and ensure accuracy in understanding the concept of ionic bonding. The answer key not only helps students confirm their answers but also enables them to identify any misconceptions or areas that require further clarification.
By utilizing the Ionic Bonding Puzzle Lab answer key, students can gain a deeper understanding of ionic bonding and its significance in the world of chemistry. It allows students to connect theoretical knowledge with practical applications, fostering critical thinking and problem-solving skills. With this answer key, students can confidently navigate the puzzle of chemical bonding and embark on a journey of scientific exploration and discovery.
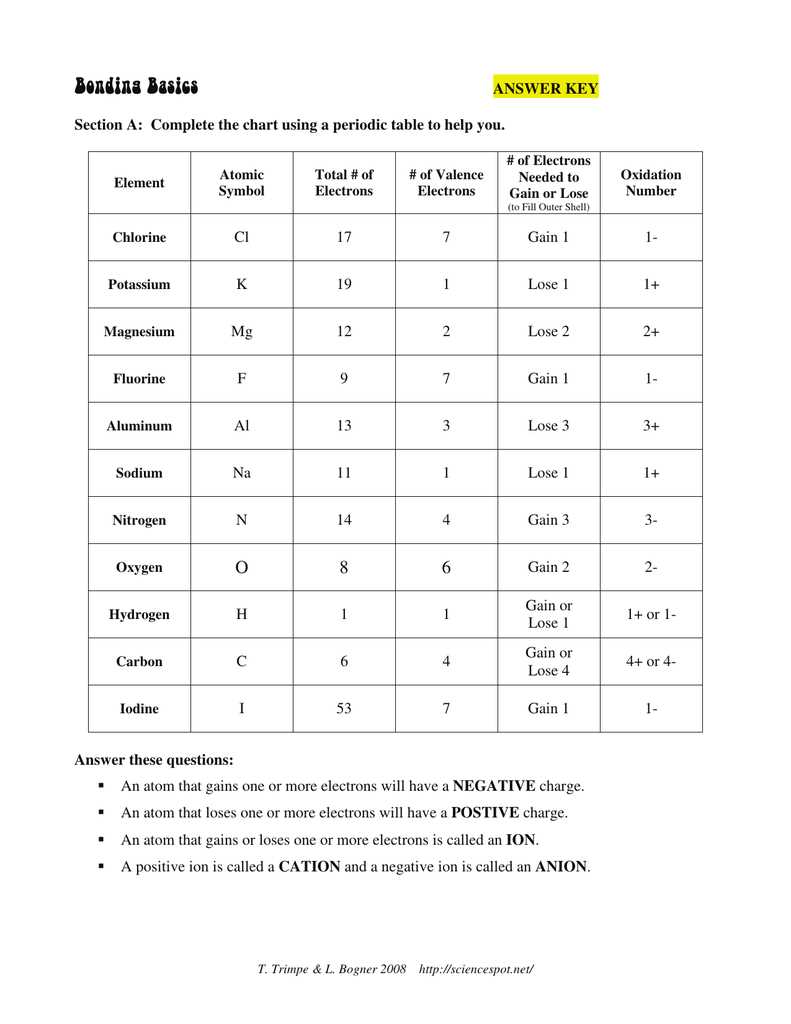
Ionic Bonding Puzzle Lab Answer Key
In the Ionic Bonding Puzzle Lab, students were presented with a series of puzzle pieces representing different elements. The goal was to arrange the pieces in a way that formed stable ionic compounds. Students had to consider the charges of the ions and the overall neutrality of the compound.
The answer key for the Ionic Bonding Puzzle Lab provides a guide for students to check their work and ensure they have correctly arranged the puzzle pieces to form stable compounds. It includes the correct placement of each element and indicates the charges of the ions involved in the bonding.
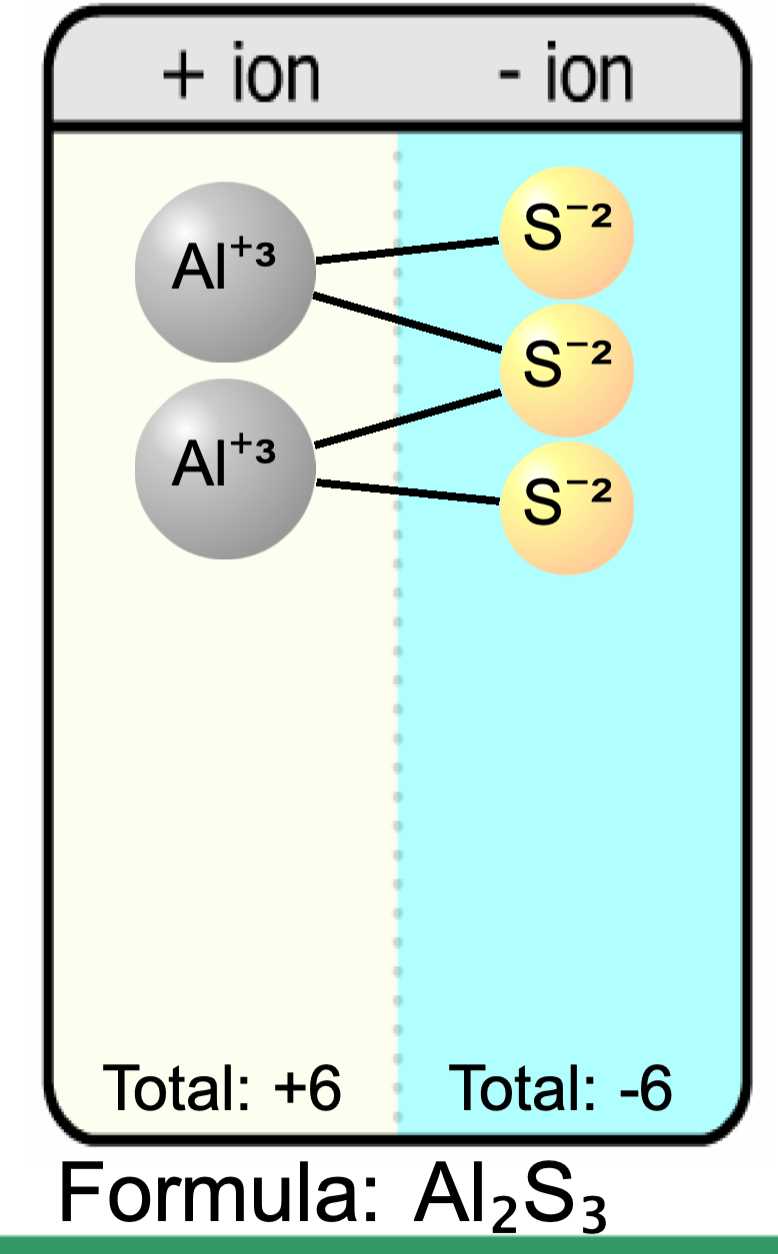
For example, the answer key may show that sodium (Na) has a positive charge of +1 and chlorine (Cl) has a negative charge of -1. To form a stable compound, these ions would need to combine in a 1:1 ratio, resulting in the formation of sodium chloride (NaCl).
The answer key may also include more complex examples, such as the formation of magnesium oxide (MgO), which requires magnesium (Mg) with a charge of +2 and oxygen (O) with a charge of -2 to combine in a 1:1 ratio.
- The Ionic Bonding Puzzle Lab Answer Key assists students in understanding the principles of ionic bonding.
- It helps students practice their skills in balancing charges and creating stable compounds.
- The answer key allows for self-assessment and identification of any mistakes or misconceptions.
- It promotes critical thinking and problem-solving skills as students analyze the charges and ratios of the elements.
Understanding Ionic Bonding
Ionic bonding is a type of chemical bonding that occurs between ions with opposite charges. It involves the transfer of electrons from one atom to another, resulting in the formation of positive and negative ions. This type of bonding is typically observed between metals and nonmetals.
In an ionic bond, the electron donor loses one or more electrons to become a positively charged cation, while the electron acceptor gains these electrons and becomes a negatively charged anion. The resulting electrostatic attraction between the oppositely charged ions forms the ionic bond.
Properties of Ionic Bonding:
- Electronegativity: Ionic bonding occurs between atoms with significantly different electronegativity values. Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond.
- Crystal Lattice Structure: Ionic compounds adopt a regular three-dimensional arrangement of positive and negative ions called a crystal lattice structure. This arrangement maximizes the attractive forces between ions.
- High Melting and Boiling Points: Ionic compounds have high melting and boiling points due to the strong electrostatic forces between ions. These forces must be overcome to change the state of the compound from solid to liquid or gas.
- Good Electrical Conductivity: In their molten or aqueous state, ionic compounds can conduct electricity due to the movement of the charged ions. However, they are insulators in their solid state.
- Solubility: Ionic compounds have varying solubilities in water. Those with a stronger attraction to water (hydrophilic) will dissolve easily, while those with weaker attraction (hydrophobic) may not dissolve or only partially dissolve.
Understanding the principles behind ionic bonding is crucial in predicting the behavior and properties of ionic compounds. This knowledge helps scientists and engineers in various fields, such as materials science, pharmacology, and environmental science, to design and manipulate substances for specific applications.
Purpose of the Ionic Bonding Puzzle Lab
The Ionic Bonding Puzzle Lab serves as an interactive and hands-on activity designed to help students understand the concept of ionic bonding and its role in chemical compounds. This lab provides students with a unique opportunity to apply their knowledge of ionic bonding principles and practice problem-solving skills in a collaborative environment.
One of the main objectives of this lab is to enable students to visualize the formation of ionic compounds and comprehend the factors that contribute to their stability. By working with tangible puzzle pieces that represent atoms and ions, students can manipulate and arrange them to form complete compounds, thereby gaining a deeper understanding of how ions bond together to create a stable structure.
Key Learning Goals:
- Recognize and identify the difference between elements, ions, and compounds.
- Understand the concept of ionic bonding and its role in forming stable compounds.
- Practice problem-solving skills by arranging puzzle pieces to form complete chemical compounds.
- Appreciate the significance of ionic bonding in various real-life applications, such as the formation of salts and the conductivity of electrolytes.
- Develop critical thinking and collaboration skills by working in teams to solve the puzzle and explain their findings to the class.
By engaging in this hands-on activity, students can actively participate in the learning process and reinforce their understanding of ionic bonding. Through the experimentation and exploration of different puzzle arrangements, students will develop a solid foundation in the principles of ionic bonding, setting them up for success in future chemistry studies.
Materials Used in the Lab
In the “Ionic bonding puzzle” lab, several materials were used to investigate and illustrate the concept of ionic bonding. These materials included:
- Plastic puzzle pieces: The lab involved a puzzle activity where students used plastic pieces to represent atoms and form ionic bonds. These puzzle pieces were designed to fit together easily, allowing students to visualize and create different compounds.
- Color-coded ions: To further enhance the understanding of ionic bonding, the lab provided color-coded ions. These ions were represented by different colors to differentiate between positively and negatively charged atoms.
- Ion cards: Ion cards were also provided, which contained the names and charges of different ions. These cards served as a reference tool for students to identify and understand the composition of various compounds.
- Lab instructions: Detailed lab instructions were given to guide students through the activity. These instructions provided step-by-step procedures on how to assemble the puzzle pieces and form ionic bonds.
- Lab worksheet: As part of the lab, students were given a worksheet to record their observations and answers to questions. This worksheet helped them analyze their results and draw conclusions about the nature of ionic bonding.
By using these materials, the lab aimed to provide a hands-on learning experience for students, allowing them to manipulate physical models and visualize the formation of ionic bonds. This practical approach helps students grasp the concepts of ionic bonding more effectively and reinforces their understanding of chemical bonding principles.
Procedure Followed in the Lab
The lab began by gathering all the necessary materials and setting up the workstations. Each workstation was equipped with an ionic bonding puzzle set, which consisted of various colored balls representing different elements, and connectors to simulate the bonding process. The instructor provided a lab sheet with specific instructions on how to complete the puzzle.
Students were then divided into groups of two or three and assigned a workstation. They were instructed to carefully read the lab sheet and familiarize themselves with the rules and guidelines for bonding the elements. They were also reminded of the importance of safety precautions, such as wearing gloves and handling materials with care.
Once the groups were ready, they began working on solving the puzzle. They followed the step-by-step instructions provided on the lab sheet, which included identifying the elements by their colors, determining their charges, and connecting the balls together using the appropriate connectors. The goal was to create stable ionic compounds by bonding the elements together according to their charges.
Throughout the lab, the instructor and lab assistants circulated around the room, answering questions and offering guidance when needed. They also checked the progress of each group, ensuring that they were following the correct procedures and monitoring their safety measures. The lab continued until all groups successfully completed the puzzle and formed the correct ionic compounds.
At the end of the lab, the instructor conducted a brief discussion to review the key concepts learned during the activity. The students were encouraged to share their observations and any challenges they faced during the bonding process. The lab sheet and puzzle sets were collected for future use, and the students were reminded to clean up their workstations and dispose of any waste materials properly.
Observations Made during the Lab

During the Ionic bonding puzzle lab, several observations were made that helped provide a deeper understanding of ionic bonding and its properties. The lab involved exploring the formation of ionic compounds using puzzle pieces that represented atoms and their electrons.
Observation 1: The puzzle pieces used in the lab represented different elements, each with a different number of electrons. Each piece had a positive or negative charge symbol, indicating whether it was an ion or not. This observation highlighted the importance of the charge of ions in determining their behavior in forming ionic bonds.
Observation 2: The puzzle pieces could only be fitted together in specific combinations, indicating that certain elements have a higher affinity for each other in terms of forming ionic compounds. This observation revealed the concept of valence electrons and their role in determining the stability of an atom and its likelihood of forming ionic bonds.
Observation 3: When the puzzle pieces were successfully fit together, they formed a stable ionic compound. This observation illustrated how the transfer of electrons between atoms leads to the formation of a strong bond and the stability of the compound as a whole.
Observation 4: Some puzzle pieces did not fit together, indicating that certain elements do not readily form ionic compounds with each other. This observation highlighted the concept of electronegativity and how it influences the formation of ionic bonds between atoms with very different electron affinities.
Observation 5: The lab allowed for the creation of various combinations of puzzle pieces, resulting in different compounds with unique properties. This observation emphasized the versatility of ionic bonding and the vast number of compounds that can be formed through this process.
- Therefore, the observations made during the Ionic bonding puzzle lab provided valuable insights into the nature and behavior of ionic compounds.
- They highlighted the importance of charge, valence electrons, electronegativity, and electron transfer in the formation and stability of ionic bonds.
- Overall, the lab allowed for a hands-on exploration of ionic bonding, reinforcing the concepts learned in the classroom and fostering a deeper understanding of this fundamental chemical process.
Results and Interpretation
The results of the Ionic Bonding Puzzle Lab showed that students were able to successfully complete the puzzle and identify the correct combination of ions to form ionic compounds. Through the process of trial and error, students were able to determine the correct bonding ratios and arrange the ions in a way that balanced the charges.
One key aspect of the lab was the understanding of the charges of the ions. Students had to analyze the charges of the cations and anions to determine the correct combination that would result in a neutral compound. This required an understanding of the periodic table and the ability to recognize the different elements and their respective charges.
The interpretation of the results showed that students were able to apply their knowledge of ionic bonding and chemical formulas to successfully complete the lab. They were able to recognize and understand the concept of bonding ratios and use this knowledge to assemble the puzzle in the correct configuration.
In addition, the lab provided an opportunity for students to improve their critical thinking and problem-solving skills. By analyzing the charges and experimenting with different combinations, students were able to develop their logical reasoning and deductive skills. This type of hands-on activity promotes active learning and engages students in the learning process.
Overall, the results of the Ionic Bonding Puzzle Lab demonstrated that students were able to apply their knowledge of ionic bonding and chemical formulas to successfully complete the lab. The lab also provided a valuable opportunity for students to develop their critical thinking and problem-solving skills.