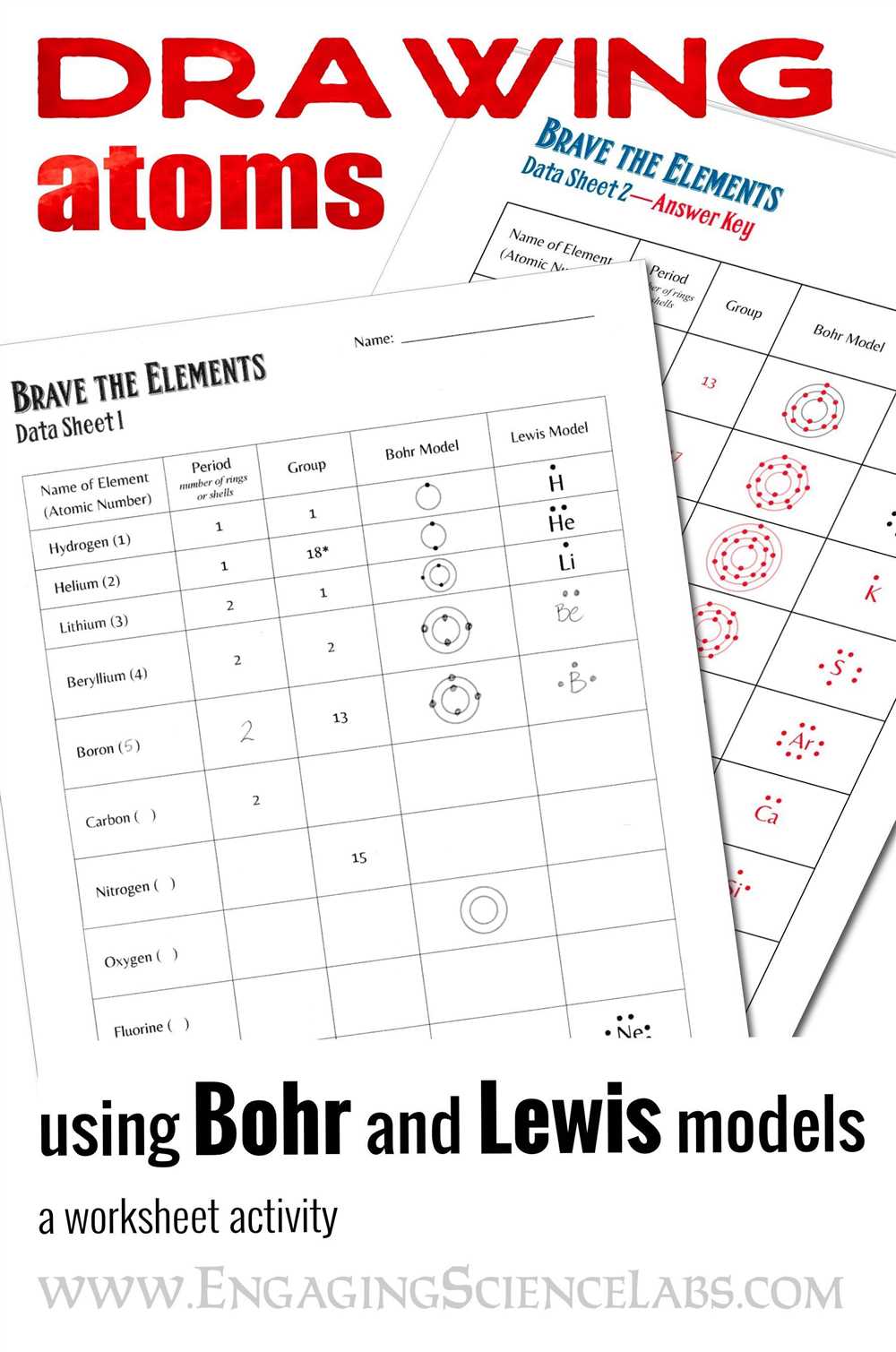
Understanding the Bohr model is essential in the field of chemistry, as it provides a visual representation of an atom’s structure and energy levels. In Bohr model practice 2, students are tasked with using their knowledge of the Bohr model to determine the electron configuration and number of valence electrons for various elements.
This answer key serves as a guide for students, providing them with the correct electron configurations and number of valence electrons for each element in the practice. By using this answer key, students can compare their answers and ensure they have a solid understanding of the Bohr model.
The Bohr model practice 2 answer key includes detailed explanations for each element, explaining how to determine the electron configuration and number of valence electrons. This allows students to not only check their answers but also learn the reasoning behind each step.
By using this answer key as a study tool, students can reinforce their understanding of the Bohr model, ensuring they are well-prepared for future applications in chemistry. With practice, students will become more confident in their ability to determine electron configurations and recognize the number of valence electrons for any given element.
Bohr Model Practice 2 A Answer Key
In the Bohr Model Practice 2 A exercise, students were given a table of elements and were asked to determine the number of protons, neutrons, and electrons for each element. Here is the answer key for the exercise:
- Element: Hydrogen (H)
- Protons: 1
- Neutrons: 0
- Electrons: 1
- Element: Helium (He)
- Protons: 2
- Neutrons: 2
- Electrons: 2
- Element: Lithium (Li)
- Protons: 3
- Neutrons: 4
- Electrons: 3
The remaining elements and their corresponding number of protons, neutrons, and electrons can be found in the provided answer key document. This exercise helps students practice their understanding of the Bohr model and the structure of atoms. By determining the number of protons, neutrons, and electrons for each element, students can better understand how these particles contribute to the overall properties of the element.
Knowing the number of protons, neutrons, and electrons is essential in understanding an element’s atomic structure, including its atomic mass, reactivity, and overall behavior. The Bohr model is a simplified representation of an atom, with electrons orbiting around a nucleus consisting of protons and neutrons. By mastering the skills needed to determine the number of protons, neutrons, and electrons, students can gain a deeper understanding of the periodic table and the fundamental building blocks of matter.
Overview of Bohr Model Practice 2 A
In Bohr Model Practice 2 A, students are given a set of atoms and asked to determine the number of protons, neutrons, and electrons present in each atom. This exercise allows students to apply their understanding of the Bohr model of the atom and practice identifying the different components of an atom.
The practice begins with a brief review of the Bohr model and its key concepts, such as the arrangement of electrons in energy levels and the relationship between the number of protons and electrons in a neutral atom. Students are then presented with a series of atoms, each represented by its atomic symbol and atomic number. Based on this information, students are tasked with determining the number of protons, neutrons, and electrons in each atom.
To solve the problems, students must recall that the atomic number of an atom indicates the number of protons, and therefore also the number of electrons in a neutral atom. They must also remember that the atomic mass of an atom is the sum of the number of protons and neutrons. By subtracting the atomic number from the atomic mass, students can calculate the number of neutrons in each atom. These calculations require students to use their knowledge of basic arithmetic and atomic structure.
A practice 2 A answer key is available to students to check their work and verify their answers. This key provides the correct number of protons, neutrons, and electrons for each atom, allowing students to compare their responses and identify any errors they may have made. By reviewing the answer key, students can gain a better understanding of the relationships between the different components of an atom and strengthen their grasp of the Bohr model and its applications.
Understanding the Bohr Model
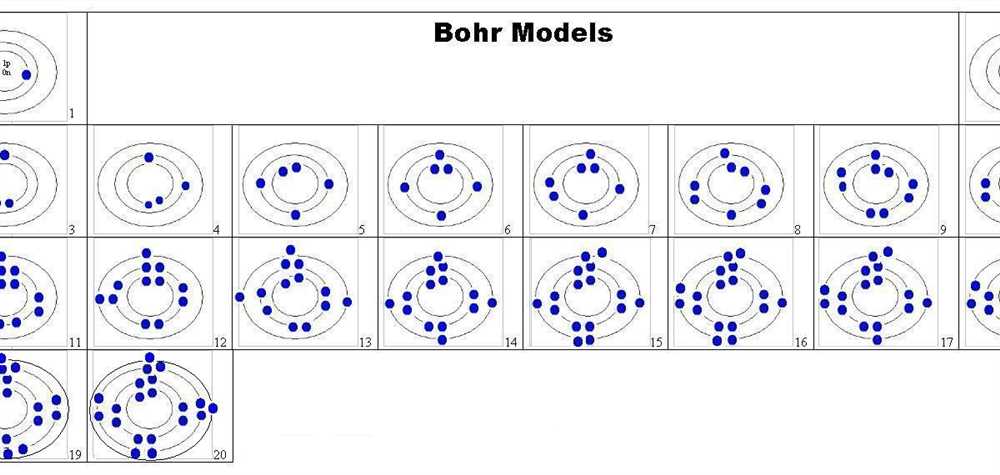
The Bohr Model, also known as the Bohr-Rutherford model, is a simplified representation of the atomic structure proposed by Danish physicist Niels Bohr in 1913. It provides a framework for understanding how electrons are arranged in an atom and how they move between energy levels. The model is based on quantized energy levels, in which electrons occupy specific orbits around the nucleus, with each orbit having a fixed energy level.
In the Bohr Model, electrons are arranged in circular orbits around the nucleus, similar to planets orbiting the sun. These orbits are also called shells or energy levels. The electrons in the innermost shell, closest to the nucleus, have the lowest energy level, while those in outer shells have higher energy levels. Electrons can move between these shells by gaining or losing energy, typically through the absorption or emission of photons.
- Key Concepts:
The Bohr Model introduces several key concepts to understanding the atomic structure:
- Energy Levels: Electrons occupy specific energy levels or shells around the nucleus.
- Orbits: These energy levels are represented by circular orbits around the nucleus.
- Quantized Energy: Electrons can only occupy certain energy levels and cannot exist between them.
- Photon Absorption and Emission: Electrons can move between energy levels by absorbing or emitting photons.
Overall, the Bohr Model helps us visualize and understand how electrons are distributed in an atom, as well as how they can change their energy states. Although it is a simplified representation, the Bohr Model laid the foundation for further developments in the field of quantum mechanics and atomic theory.
The Importance of Practice Exercises
Practice exercises are a critical component of learning and mastering any subject, including the Bohr model in chemistry. They provide an opportunity for students to apply their knowledge and skills in a practical way, allowing them to strengthen their understanding and retention of the material. By actively engaging with the content through practice exercises, students can identify areas where they may be struggling and focus their efforts on improving those specific areas.
Practice exercises also help students develop problem-solving skills. They encourage critical thinking and analytical reasoning, as students must analyze the given information, identify the relevant concepts, and apply them to solve a particular problem. This process not only enhances their understanding of the subject matter but also prepares them for real-life scenarios where problem-solving skills are crucial.
Regular practice exercises can improve students’ confidence in their abilities. When students consistently engage in practice exercises, they gain a sense of familiarity and mastery over the material. This confidence can have a positive impact on their overall performance and achievement in the subject. It also helps them approach challenging topics with a more positive and determined mindset, knowing that with practice and effort, they can overcome any difficulties.
Moreover, practice exercises provide valuable feedback and assessment opportunities. By attempting practice exercises, students can receive feedback on their performance and identify areas that require further improvement. This feedback loop allows them to track their progress, make necessary adjustments to their study strategies, and focus on areas of weakness. Practice exercises also serve as an assessment tool for both students and instructors, helping them gauge the level of understanding and mastery achieved.
In conclusion, practice exercises play a crucial role in the learning process. They facilitate understanding, develop problem-solving skills, boost confidence, and provide valuable feedback. By incorporating regular practice exercises into their study routine, students can enhance their learning outcomes and achieve success in mastering complex subjects like the Bohr model.
How to Use the Answer Key
In order to effectively use the answer key for the Bohr model practice 2, it is important to follow a few key steps. By following these steps, you will be able to check your answers, identify any mistakes, and improve your understanding of the topic.
1. Complete the Practice Questions: Before using the answer key, make sure you have completed all the practice questions in the Bohr model practice 2 worksheet. This will ensure that you have attempted each question to the best of your ability.
2. Compare Your Answers: Once you have completed the practice questions, compare your answers to the ones provided in the answer key. Pay close attention to any differences or inconsistencies between your answers and the correct ones.
3. Identify Mistakes: As you compare your answers, make note of any mistakes you have made. Try to understand why you made those mistakes and what concepts or principles you may need to review or study further.
4. Seek Clarification: If you are unsure about why a particular answer is correct or if you have any questions about the material, don’t hesitate to seek clarification. You can consult your teacher, a classmate, or refer to your textbook or other reliable resources for further information.
5. Learn from Your Mistakes: Use the mistakes you have identified as an opportunity to improve your understanding of the Bohr model and related concepts. Take the time to review the relevant material, ask questions, and practice similar problems until you feel confident in your understanding.
6. Retake the Practice Questions: Once you have gone through the answer key and addressed any mistakes or areas of confusion, it may be helpful to retake the practice questions. This will allow you to apply what you have learned and solidify your understanding of the Bohr model.
7. Reflect on Your Progress: After using the answer key and going through the practice questions again, take a moment to reflect on your progress. Consider how much you have improved and what areas you still need to work on. Use this reflection to inform your future studying and practice.
By following these steps and using the answer key effectively, you can enhance your understanding of the Bohr model and improve your performance on related assessments and assignments.
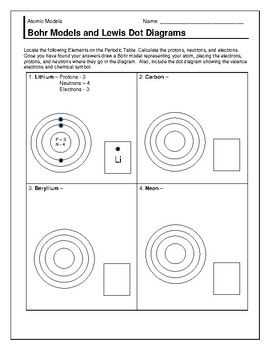
Answer Key for Question 1
Question: Draw the Bohr model for the following elements:
- a) Neon (Ne)
- b) Calcium (Ca)
- c) Oxygen (O)
Answer:
a) Neon (Ne):
In the Bohr model of the Neon atom, there are 10 protons in the nucleus and 10 electrons in the energy levels surrounding the nucleus. The first energy level has 2 electrons, the second energy level has 8 electrons, and the third energy level has 2 electrons. The diagram of the Bohr model for Neon would show a nucleus with 10 protons and 10 neutrons, and three concentric circles representing the energy levels with the corresponding number of electrons distributed around the circles.
b) Calcium (Ca):
In the Bohr model of the Calcium atom, there are 20 protons in the nucleus and 20 electrons in the energy levels surrounding the nucleus. The first energy level has 2 electrons, the second energy level has 8 electrons, the third energy level has 8 electrons, and the fourth energy level has 2 electrons. The diagram of the Bohr model for Calcium would show a nucleus with 20 protons and 20 neutrons, and four concentric circles representing the energy levels with the corresponding number of electrons distributed around the circles.
c) Oxygen (O)
In the Bohr model of the Oxygen atom, there are 8 protons in the nucleus and 8 electrons in the energy levels surrounding the nucleus. The first energy level has 2 electrons and the second energy level has 6 electrons. The diagram of the Bohr model for Oxygen would show a nucleus with 8 protons and 8 neutrons, and two concentric circles representing the energy levels with the corresponding number of electrons distributed around the circles.
Answer Key for Question 2
Question 2 in the Bohr model practice worksheet asks students to determine the number of electrons in the second energy level of various elements. The correct answers are as follows:
Lithium (Li): Lithium has an atomic number of 3, which means it has 3 electrons. Following the rule that the second energy level can hold a maximum of 8 electrons, we can determine that lithium has 2 electrons in its second energy level.
Cobalt (Co): Cobalt has an atomic number of 27, which means it has 27 electrons. To determine the number of electrons in the second energy level, we need to consider the electron configuration of cobalt. The electron configuration of cobalt is 2-8-15-2, indicating that cobalt has 2 electrons in its second energy level.
- Magnesium (Mg): Magnesium has an atomic number of 12, which means it has 12 electrons. The electron configuration of magnesium is 2-8-2, indicating that magnesium has 2 electrons in its second energy level.
- Oxygen (O): Oxygen has an atomic number of 8, which means it has 8 electrons. The electron configuration of oxygen is 2-6, indicating that oxygen has 6 electrons in its second energy level.
- Silicon (Si): Silicon has an atomic number of 14, which means it has 14 electrons. The electron configuration of silicon is 2-8-4, indicating that silicon has 4 electrons in its second energy level.
These are the correct answers for Question 2 in the Bohr model practice worksheet. By understanding the electron configuration and the maximum number of electrons each energy level can hold, students can accurately determine the number of electrons in the second energy level of various elements.