When it comes to understanding the structure of molecules, Lewis structures are an essential tool. This worksheet provides a set of practice problems to help you master the skill of creating Lewis structures and understanding the geometry and bonding of molecules.
In this worksheet, you will find a series of molecules for which you need to draw Lewis structures. By following a set of rules and guidelines, you will be able to determine the correct placement of atoms and electrons in each molecule. Through this process, you will gain a deeper understanding of how different elements interact and bond with one another.
Once you have completed the worksheet, the answers provided will help you check your work and ensure that you have accurately represented the structure of each molecule. Additionally, the answers will explain the rationale behind each structure, giving you insights into the principles and concepts of Lewis structures.
Lewis Structure Worksheet 1 Answers
In this Lewis Structure Worksheet 1, you will find the answers to various questions related to Lewis structures, which are diagrams that show the bonding between atoms in a molecule and the lone pairs of electrons that may exist in the molecule. These diagrams are useful for understanding the molecular structure and predicting the chemical properties of compounds.
Below are the answers to some of the questions in the worksheet:
- Question 1: Draw the Lewis structure for the compound “CO2”.
- Question 2: Determine the number of valence electrons in the compound “H2O”.
- Question 3: Identify the molecular shape of the compound “CH4”.
- Question 4: Draw the Lewis structure for the compound “NH3”.
- Question 5: Explain the concept of formal charge in Lewis structures.
The Lewis structure for CO2 shows that the carbon atom is double-bonded to both oxygen atoms. The carbon atom has no lone pairs of electrons, while each oxygen atom has two lone pairs.
The Lewis structure for H2O suggests that there are 8 valence electrons in the compound. The oxygen atom has 6 valence electrons, and each hydrogen atom has 1 valence electron.
The Lewis structure for CH4 indicates that the molecule has a tetrahedral shape, with the carbon atom in the center and the four hydrogen atoms surrounding it.
The Lewis structure for NH3 shows that the nitrogen atom is bonded to three hydrogen atoms. The nitrogen atom also has one lone pair of electrons.
Formal charge is a measure of the distribution of electrons in a molecule. It is calculated by subtracting the number of lone pair electrons and half of the bonding electrons from the number of valence electrons of an atom. A Lewis structure with the lowest formal charges on each atom is considered to be the most stable.
These are just a few examples of the answers you will find in the Lewis Structure Worksheet 1. By practicing drawing Lewis structures and understanding their properties, you will develop a solid foundation in chemical bonding and molecular structure.
Understanding Lewis Structures
Lewis structures are a helpful tool in understanding the arrangement of electrons within molecules. They provide a simplified representation that allows chemists to visualize the bonding and electron distribution in a molecule.
What are Lewis structures?
Lewis structures are diagrams that show the bonding between atoms in a molecule, as well as the lone pairs of electrons that are not involved in bonding. They are named after Gilbert N. Lewis, who introduced this method in 1916. In a Lewis structure, each atom is represented by its atomic symbol, and the valence electrons are shown as dots or lines surrounding the atoms.
How to draw Lewis structures?
To draw a Lewis structure, you need to know the number of valence electrons for each atom in the molecule. The valence electrons are the electrons in the outermost energy level of an atom. The total number of valence electrons in a molecule is equal to the sum of the valence electrons of all atoms. Once you have determined the total number of valence electrons, you can start placing them around the atoms, following certain rules:
- Each atom should have an octet of electrons, except for hydrogen, which only needs two electrons to achieve stability.
- Place a single bond between the atoms, using two electrons.
- If there are remaining electrons, place them as lone pairs around the atoms.
Why are Lewis structures important?
Lewis structures are important because they help us understand the chemical behavior of molecules. By visualizing the arrangement of electrons, we can predict the shape of the molecule and its polarity. Lewis structures also provide a basis for understanding chemical reactions, as they show how electrons are transferred or shared between atoms.
In conclusion, Lewis structures are a valuable tool in chemistry that allow us to visualize the arrangement of electrons in molecules. They provide insights into the bonding and electron distribution within a molecule, helping us understand its chemical properties and behavior.
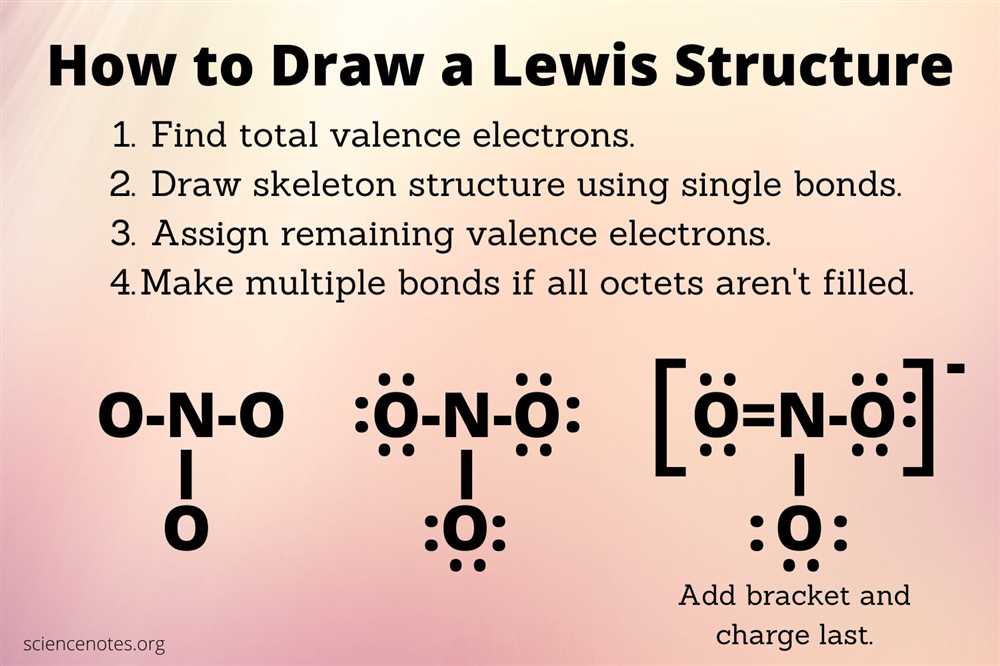
Steps to Drawing Lewis Structures
A Lewis structure is a visual representation of the electron arrangement in a molecule or ion. It helps us understand the bonding and non-bonding electrons in a compound and predict its molecular geometry. To draw a Lewis structure, follow these steps:
Step 1: Count the total number of valence electrons
Start by determining the number of valence electrons for each atom in the compound. Valence electrons are the electrons in the outermost energy level of an atom. The group number of an element on the periodic table indicates the number of valence electrons it has. For example, carbon in Group 14 has 4 valence electrons.
Step 2: Identify the central atom
In many compounds, one atom plays a central role and forms the backbone of the molecule. Usually, the least electronegative element is the central atom. The exception is hydrogen, which is rarely the central atom. Once you identify the central atom, place it in the center of your Lewis structure.
Step 3: Connect the atoms with single bonds
Using dots or lines, connect the central atom to the surrounding atoms. Each bond requires two electrons, so the total number of electrons used for bonds should be subtracted from the total number of valence electrons determined in Step 1.
Step 4: Distribute the remaining electrons
After accounting for the electrons used in bonds, distribute the remaining electrons around the atoms to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to acquire a full set of eight valence electrons, providing them with greater stability.
Step 5: Check the formal charges
Formal charges help determine the most stable Lewis structure. To calculate formal charges, compare the number of valence electrons an atom has in its neutral state to the number of valence electrons it has in the Lewis structure. Try to minimize formal charges or distribute them in such a way that the overall charge of the molecule or ion is neutral.
By following these steps, you can create accurate Lewis structures that depict the electron arrangement in compounds and help predict their properties and behaviors.
Lewis Structures for Simple Molecules

When studying chemistry, one of the fundamental skills is the ability to draw Lewis structures for simple molecules. Lewis structures are diagrams that show the arrangement of atoms in a molecule, as well as the placement of electrons. By following a few rules and guidelines, it is possible to determine the Lewis structure of almost any molecule.
A Lewis structure consists of the chemical symbol of each atom in the molecule, with lines representing bonds between them. The valence electrons of each atom are also shown. These are the electrons involved in bonding and are typically depicted as dots around the chemical symbol.
For example, let’s consider the molecule water (H2O). The Lewis structure of water shows two hydrogen atoms (H) bonded to one oxygen atom (O). The oxygen atom has two unpaired electrons, which are represented as dots. The hydrogen atoms form single bonds with the oxygen atom, resulting in a bent molecular shape.
Another example is carbon dioxide (CO2). The Lewis structure of carbon dioxide shows one carbon atom (C) bonded to two oxygen atoms (O). The carbon atom has four valence electrons, while each oxygen atom has six valence electrons. To satisfy the octet rule, which states that atoms tend to gain, lose, or share electrons until they have a full outer shell of eight electrons, the carbon atom forms double bonds with each oxygen atom.
In conclusion, understanding how to draw Lewis structures for simple molecules is essential for understanding the chemical properties and behavior of various substances. By following specific guidelines and taking into account the valence electrons of each atom, it is possible to accurately represent the arrangement of atoms and electrons in a molecule.
Lewis Structures for Polyatomic Ions
The Lewis structure for a polyatomic ion is a representation of how the atoms are bonded together and the placement of electrons within the ion. Polyatomic ions are groups of atoms that carry a charge and act as a single unit in chemical reactions. Understanding the Lewis structures of polyatomic ions is important in predicting their reactivity and understanding their role in chemical reactions.
When determining the Lewis structure of a polyatomic ion, it is important to consider the total number of valence electrons in the ion. Valence electrons are the outermost electrons of an atom and are involved in bonding. The Lewis structure should show all of the valence electrons and the placement of the atoms within the ion.
One helpful approach to determining the Lewis structure of a polyatomic ion is to follow a step-by-step process. First, calculate the total number of valence electrons by adding up the valence electrons of each atom in the ion. Second, determine the central atom of the ion based on its importance in the structure. The central atom is usually the atom that forms the most bonds in the ion. Third, draw a skeletal structure of the ion, showing the bonds between the atoms. Fourth, distribute the remaining electrons around the atoms, placing them in pairs to satisfy the octet rule for each atom. Finally, check the formal charges of the atoms to ensure they are minimized.
It is important to note that not all polyatomic ions follow the octet rule. Some ions, such as the sulfate ion (SO₄²⁻), have expanded octets, meaning they can accommodate more than eight electrons around the central atom. These ions may require more complex Lewis structures to accurately represent the placement of electrons in the ion.
Overall, Lewis structures for polyatomic ions provide a visual representation of how the atoms are bonded and the placement of electrons within the ion. Understanding these structures allows for a better understanding of the reactivity and behavior of polyatomic ions in chemical reactions.
Lewis Structures for Compounds with Double and Triple Bonds
Lewis structures provide a visual representation of the bonding and electron distribution in a molecule. When dealing with compounds that contain double or triple bonds, it is important to understand how to properly represent these bonds in a Lewis structure.
Double bonds occur when two pairs of electrons are shared between two atoms. To represent a double bond in a Lewis structure, a double line is drawn between the two atoms, and two pairs of electrons are placed between them. For example, in the Lewis structure of carbon dioxide (CO2), the carbon atom is double bonded to each oxygen atom, resulting in a total of four shared electrons.
Triple bonds occur when three pairs of electrons are shared between two atoms. To represent a triple bond in a Lewis structure, a triple line is drawn between the two atoms, and three pairs of electrons are placed between them. For example, in the Lewis structure of nitrogen gas (N2), the two nitrogen atoms are triple bonded to each other, resulting in a total of six shared electrons.
When drawing Lewis structures for compounds with double and triple bonds, it is important to consider the octet rule. The atoms involved in the double or triple bond may have less than an octet of electrons, while the other atoms in the molecule should have a complete octet. Additionally, lone pairs of electrons may be present on atoms other than those involved in the multiple bond.
In summary, Lewis structures provide a visual representation of the bonding and electron distribution in a molecule. When dealing with compounds that contain double or triple bonds, it is important to properly represent these bonds using double and triple lines, while considering the octet rule and the presence of lone pairs of electrons.
Practice Problems for Drawing Lewis Structures
In order to understand the structure of molecules, it is important to be able to draw Lewis structures. These diagrams show the arrangement of atoms and electrons in a molecule, helping us to understand its shape, bonding, and reactivity. Here are some practice problems to help you improve your skills in drawing Lewis structures.
Problem 1:
Draw the Lewis structure for carbon dioxide (CO2).
- Identify the central atom: In this case, carbon is the central atom because it is less electronegative than oxygen.
- Count the total number of valence electrons: Carbon has 4 valence electrons, and each oxygen atom has 6 valence electrons, so the total is 4 + 2(6) = 16 electrons.
- Connect the atoms: Carbon forms double bonds with both oxygen atoms, using 4 electrons. Each oxygen atom uses 2 electrons to form a bond with carbon. This leaves 4 electrons left.
- Distribute the remaining electrons: Place the remaining electrons around the atoms to satisfy the octet rule. Each oxygen atom needs 2 more electrons to have a full octet, so the remaining 4 electrons will be placed on the oxygen atoms.
| O=C=O |
Problem 2:
Draw the Lewis structure for ammonia (NH3).
- Identify the central atom: Nitrogen is the central atom because it is less electronegative than hydrogen.
- Count the total number of valence electrons: Nitrogen has 5 valence electrons, and each hydrogen atom has 1 valence electron, so the total is 5 + 3(1) = 8 electrons.
- Connect the atoms: Nitrogen forms single bonds with each hydrogen atom, using 3 electrons. This leaves 5 electrons left.
- Distribute the remaining electrons: Place the remaining electrons around the atoms to satisfy the octet rule. Each hydrogen atom needs 1 more electron to have a full octet, so the remaining 5 electrons will be placed on the hydrogen atoms.
| H |
| H-N-H |
By practicing these problems, you will become more familiar with the process of drawing Lewis structures and improve your ability to understand the structure of molecules.