
Understanding acids, bases, and salts is essential in the field of chemistry. These three types of substances play crucial roles in various chemical reactions and are fundamental to our understanding of the world around us. In this article, we will provide an overview of the answers to a worksheet that focuses on acids, bases, and salts.
Acids are substances that can donate protons, or H+ ions, in a chemical reaction. They have a sour taste and can cause a burning sensation on the skin. One of the key concepts covered in the worksheet is the pH scale, which measures the acidity or basicity of a substance. Acids have a pH below 7, with lower numbers indicating a higher concentration of H+ ions. The worksheet may include questions about identifying acids and calculating their pH values.
Bases, on the other hand, are substances that can accept protons, or H+ ions, in a chemical reaction. They have a bitter taste and a slippery feel. Bases have a pH above 7, with higher numbers indicating a higher concentration of OH- ions. The worksheet may ask students to identify bases and calculate their pH values. It may also cover the concept of neutralization, where an acid reacts with a base to form water and a salt.
Salts are the products of the neutralization reaction between an acid and a base. They are formed when the H+ ions from the acid combine with the OH- ions from the base. Salts can be either acidic, basic, or neutral, depending on the nature of the acid and base involved. The worksheet may include questions about the properties of different salts and their formation.
Studying acids, bases, and salts is crucial for understanding the principles of chemistry and their applications in various fields. The worksheet on acids, bases, and salts provides students with an opportunity to reinforce their knowledge and test their understanding of these important concepts. By answering the questions on the worksheet, students can gain a deeper understanding of the properties and behavior of acids, bases, and salts, and their role in chemical reactions.
What are acids, bases, and salts?
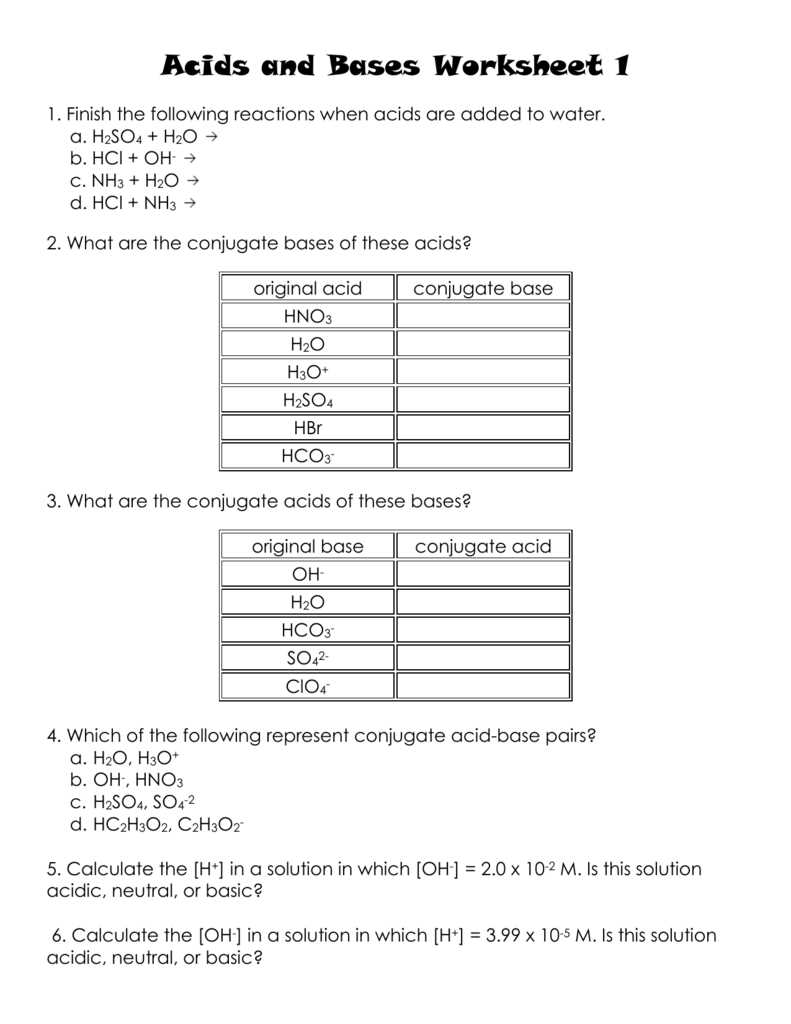
Acids, bases, and salts are substances that play important roles in chemistry. They are classified based on their chemical properties and reactions.
Acids, by definition, are substances that can donate hydrogen ions (H+) when dissolved in water. They have a sour taste and can cause a burning sensation on skin or mucous membranes. Acids can react with metals, carbonates, and bases to form salts. Examples of common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and citric acid (C6H8O7).
Bases, on the other hand, are substances that can accept hydrogen ions (H+) or donate hydroxide ions (OH-) when dissolved in water. They have a bitter taste, a soapy feel, and can cause a slippery sensation. Bases can neutralize acids and produce salts. Examples of common bases include sodium hydroxide (NaOH), ammonia (NH3), and baking soda (NaHCO3).
Salts are the result of a reaction between an acid and a base. They are ionic compounds that are formed when the hydrogen ion from the acid is replaced by a metal ion or another positive ion. Salts are usually solid at room temperature and can be dissolved in water to form an electrolyte solution. Examples of common salts include sodium chloride (NaCl), calcium carbonate (CaCO3), and potassium iodide (KI).
In summary, acids donate hydrogen ions, bases accept or donate hydrogen ions, and salts are the result of a reaction between an acid and a base. Understanding the properties and reactions of acids, bases, and salts is essential in many areas of chemistry and everyday life.
Acid
An acid is a chemical substance that has a pH value less than 7. It is characterized by its ability to donate hydrogen ions (H+) in an aqueous solution. Acids are commonly found in everyday life, such as citrus fruits like lemons and oranges, vinegar, and stomach acid. They can also be produced through chemical reactions, such as the reaction between a metal and an acid.
Properties of Acids:
- Acids have a sour taste.
- They can cause a burning sensation on the skin and in the eyes.
- Acids react with metals to produce hydrogen gas.
- They turn blue litmus paper red.
- Acids can conduct electricity when dissolved in water.
Common Acids:
| Acid | Chemical Formula | Source |
|---|---|---|
| Hydrochloric acid | HCl | Stomach acid |
| Sulfuric acid | H2SO4 | Industrial production |
| Acetic acid | CH3COOH | Vinegar |
| Citric acid | C6H8O7 | Citrus fruits |
Acids play a crucial role in various chemical reactions and industries. They are used in food production, cleaning products, and even in the production of pharmaceuticals. Understanding the properties and behavior of acids is essential in the study of chemistry and its applications.
Base

A base is a substance that can accept a proton or donate an electron pair. Bases are commonly referred to as alkaline substances. They have a pH greater than 7 and can neutralize acids by reacting with them to form water and a salt. Bases are essential in many chemical reactions and are used in various industries and applications.
One common example of a base is sodium hydroxide (NaOH), also known as caustic soda. It is a strong base that is used in the production of soaps, detergents, and various chemicals. Sodium hydroxide can react with acids to form water and a salt, neutralizing the acidic properties.
Bases can be classified into two categories: strong bases and weak bases. Strong bases completely dissociate in water, while weak bases only partially dissociate. An example of a strong base is potassium hydroxide (KOH), which is often used in the production of fertilizers and dyes. Ammonia (NH3) is an example of a weak base, commonly used in household cleaning products.
- Bases have a pH greater than 7.
- They can accept a proton or donate an electron pair.
- Common bases include sodium hydroxide and potassium hydroxide.
- They can neutralize acids to form water and a salt.
- Bases are used in various industries and applications.
In summary, bases are alkaline substances that can accept a proton or donate an electron pair. They have a pH greater than 7 and are important in many chemical reactions. Whether it’s in the production of chemicals, cleaning products, or other applications, bases play a crucial role in various industries.
Salt
Salt is a chemical compound that is formed when an acid reacts with a base. It is composed of positive ions, called cations, and negative ions, called anions. The cation is typically a metal, such as sodium or potassium, while the anion is derived from an acid, such as chloride or sulfate.
Salt is an essential part of our daily lives. It is used for various purposes, including seasoning food, preserving and flavoring in cooking, and as a condiment. It is also used in many industrial processes, such as the production of paper, glass, and detergents. In addition, salt has been used for centuries for its medicinal properties, including its ability to treat wounds and infections.
When salt dissolves in water, it dissociates into its constituent ions, which can conduct electricity. This property is utilized in various applications, such as in electrolysis and in the generation of electricity in saltwater batteries. Salt also plays an important role in the electrolyte balance of our bodies, as it helps to maintain fluid balance and regulate nerve and muscle function.
In conclusion, salt is a versatile compound that is used in numerous industries and has various applications. Its unique properties make it an indispensable part of our daily lives and its importance extends far beyond its use as a common seasoning.
Properties of acids, bases, and salts
Acids, bases, and salts are important substances that play a significant role in various chemical processes. They have distinct properties that set them apart from each other.
Acids are substances that donate hydrogen ions (H+) in a chemical reaction. They have a sour taste and can react with metals to produce hydrogen gas. Acids also turn blue litmus paper red and have a pH value less than 7. Some common examples of acids include hydrochloric acid, sulfuric acid, and acetic acid.
Bases, on the other hand, are substances that accept hydrogen ions (H+) in a chemical reaction. They have a bitter taste and a slippery feel. Bases turn red litmus paper blue and have a pH value greater than 7. Examples of bases include sodium hydroxide, potassium hydroxide, and ammonia.
Salts are compounds formed when an acid reacts with a base. They are usually formed through a process called neutralization, where the hydrogen ions from the acid combine with hydroxide ions from the base to form water. Salts are often crystalline solids and can conduct electricity when dissolved in water. Some common examples of salts include sodium chloride, calcium sulfate, and potassium nitrate.
In summary, acids, bases, and salts have distinctive properties that allow us to identify and differentiate them. Acids donate hydrogen ions, have a sour taste, and turn blue litmus paper red. Bases accept hydrogen ions, have a bitter taste, and turn red litmus paper blue. Salts are formed through the neutralization of an acid and a base, and they can conduct electricity when dissolved in water.
Acidic properties

An acid is a chemical compound that donates hydrogen ions (H+) or protons to a solution, making it acidic. Acids have certain properties that can help identify them. One property is their sour taste, which is why many acids are referred to as “sour”. Another property is their ability to turn blue litmus paper red. These properties are a result of the high concentration of hydrogen ions in acidic solutions.
Acids are classified into two categories: strong acids and weak acids. Strong acids completely ionize in water, meaning they completely dissociate into hydrogen ions and anions. Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). Weak acids, on the other hand, only partially ionize in water. They do not completely dissociate and only release a small number of hydrogen ions. Acetic acid (CH3COOH) and carbonic acid (H2CO3) are examples of weak acids.
In terms of pH, acids have a pH value less than 7. The lower the pH value, the stronger the acid. The pH scale ranges from 0 to 14, with 7 being neutral. Acids have a pH value closer to 0, while bases have a pH value closer to 14. It is important to note that the pH scale is logarithmic, meaning each unit represents a tenfold difference in acidity or basicity.
- Acids can react with metals to produce hydrogen gas.
- They can neutralize bases to form water and a salt.
- They can also react with carbonates to produce carbon dioxide gas.
Overall, acidic properties are an important aspect of chemistry. Understanding acids and their properties helps in various fields, including biology, medicine, and environmental science. Acid-base reactions are fundamental to many chemical processes, and knowing how and when acids behave can be crucial in numerous applications.
Basic Properties
Acidic and basic properties play an important role in chemistry. Acids are substances that can donate a hydrogen ion (H+) to another substance, while bases are substances that can accept a hydrogen ion. Acids typically have a sour taste and can cause a burning sensation on the skin, while bases have a bitter taste and can feel slippery.
One of the key properties of acids is their ability to react with metals to produce hydrogen gas. This is known as the metal-acid reaction. For example, when hydrochloric acid (HCl) reacts with zinc (Zn), it produces zinc chloride (ZnCl2) and hydrogen gas (H2).
Bases, on the other hand, have the ability to neutralize acids. This is known as the acid-base reaction. When an acid and a base react, they form water and a salt. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it forms water (H2O) and sodium chloride (NaCl).
Key Points:
- Acids donate hydrogen ions, while bases accept hydrogen ions.
- Acids have a sour taste and can cause a burning sensation, while bases have a bitter taste and can feel slippery.
- Acids react with metals to produce hydrogen gas.
- Bases neutralize acids and form water and a salt.
Salty properties
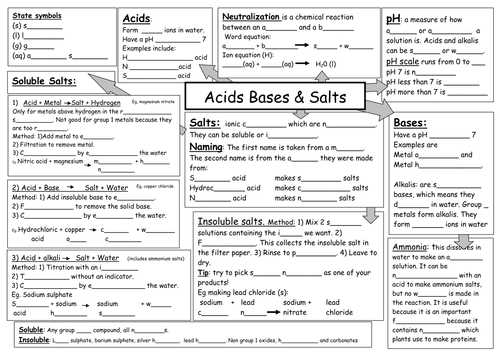
In chemistry, salts are a fundamental group of compounds that exhibit a variety of properties. Salts are formed when an acid reacts with a base, resulting in the formation of ions. These ions then combine to form a solid substance, which we commonly refer to as salt. Salts can come in many different forms, from common table salt (sodium chloride) to more complex compounds like magnesium sulfate or calcium carbonate.
1. Solubility: One of the key properties of salts is their solubility in water. Some salts are highly soluble, meaning they dissolve easily in water, while others are insoluble and do not dissolve. This property is important in various processes, such as the crystallization of salts or the separation of mixtures.
2. Conductivity: Salts are good conductors of electricity when dissolved in water. This is because the dissolved salts dissociate into ions, which can carry an electric charge and allow for the flow of current. This property is utilized in various applications, from electrolysis to the functioning of batteries.
3. Taste: Salts are well-known for their characteristic taste. However, the taste can vary depending on the specific salt. For example, sodium chloride (table salt) is salty, while potassium chloride has a slightly bitter taste. The taste of salts is often used to enhance the flavor of food and can even be detected in small quantities.
4. Melting and boiling point: Salts generally have higher melting and boiling points compared to other compounds. This is due to the strong ionic bonds between their constituent ions. The high melting and boiling points make salts useful in various industrial processes, such as the production of metals or the manufacturing of glass.
5. pH: Salts can be acidic, basic, or neutral, depending on the acids and bases from which they are formed. Acidic salts release hydrogen ions (H+) when dissolved in water, while basic salts release hydroxide ions (OH-). Neutral salts do not have a significant influence on the pH of a solution. Understanding the pH properties of salts is essential in various fields, including medicine and environmental science.
In conclusion, salts possess unique and diverse properties, making them essential in various aspects of chemistry and everyday life. Their solubility, conductivity, taste, melting and boiling points, and pH characteristics allow for a range of applications and contribute to our understanding of chemical reactions and their effects.