When working with numbers in science and mathematics, it is essential to understand the concept of significant figures and precision. Significant figures represent the precision or the number of digits that are known with confidence in a measurement or calculation. They indicate the level of uncertainty in a value and are crucial in communicating the reliability of data.
For homework problems that involve significant figures and precision, you need to carefully consider the rules for determining the number of significant figures in a given value. The general rule states that all non-zero digits are considered significant, zeros between non-zero digits are significant, and leading zeros are not significant. Trailing zeros are significant only if they are after a decimal point or are explicitly indicated with a decimal point and a significant figure in front.
When providing answers to homework problems involving significant figures and precision, it is important to adhere to the rules and convey the appropriate level of precision. Your answers should reflect the same number of significant figures as the input values or the least number of significant figures in the problem. Additionally, you should round or truncate your final answer to match the proper number of significant figures required.
By understanding the concept of significant figures and precision, and applying the rules accurately in your homework answers, you will demonstrate a solid grasp of the fundamental principles in scientific measurements and calculations. This attention to detail will not only earn you points in your assignments but also equip you with a valuable skill set for future scientific endeavors.
Significant Figures and Precision Homework Answers
In the study of science, accuracy and precision are two important concepts to understand. When answering homework questions that require determining significant figures and precision, it is crucial to consider the rules and guidelines associated with these concepts.
Significant figures, also known as significant digits, represent the meaningful and reliable digits in a measured or calculated value. These figures indicate the precision of a measurement or calculation and are used to convey the level of confidence in the result. When determining the number of significant figures in a value, certain rules apply:
- Non-zero digits are always significant.
- Leading zeroes (zeros before any non-zero digit) are not significant.
- Captive zeroes (zeros between non-zero digits) are significant.
- Trailing zeroes (zeros after the decimal point and after any non-zero digits) are significant.
- Zeros used solely for spacing the decimal point are not significant.
Precision refers to the level of detail or exactness in a measurement or calculation. It is often determined by the number of significant figures present in a value. The greater the number of significant figures, the greater the precision. For example, a value with three significant figures is more precise than a value with only two significant figures.
To answer homework questions related to significant figures and precision, one must carefully follow these rules and guidelines. It is important to identify and count the significant figures in a given value, as well as use the appropriate number of significant figures in any calculations or final answers. By doing so, one can ensure accuracy and demonstrate an understanding of the concepts of significant figures and precision in science.
What are significant figures?
Significant figures, also known as significant digits, are the digits in a number that carry meaningful information. They represent the precision or accuracy of a measurement or calculation. In other words, significant figures indicate the level of certainty in a number.
When expressing a number, significant figures include all the certain digits and one uncertain digit. The uncertain digit is the last digit in the number, which is estimated or interpolated based on the precision of the measurement device. Significant figures help communicate the level of precision in scientific measurements and calculations.
In general, there are a few rules to determine the number of significant figures in a given number. Non-zero digits are always significant, zeros between non-zero digits are also considered significant, and trailing zeros to the right of the decimal point are significant. However, leading zeros (zeros to the left of the first non-zero digit) are not considered significant unless there is a decimal point present.
To illustrate, let’s consider the number 345.0. Here, all three digits (3, 4, and 5) are significant, along with the zero after the decimal point. Therefore, this number has four significant figures. On the other hand, a number like 0.0034 only has two significant figures, as the leading zeros are not considered significant. Understanding significant figures is crucial for accurately representing the precision and uncertainty in scientific measurements and calculations.
How to determine the number of significant figures in a measurement?
When measuring physical quantities, it is important to determine the number of significant figures in order to reflect the precision and accuracy of the measurement. The number of significant figures indicates the reliability and confidence level of a measurement.
To determine the number of significant figures in a measurement, there are several rules to follow:
- Non-zero digits: All non-zero digits are always considered significant. For example, in the measurement 34.7 cm, there are three significant figures.
- Leading zeros: Leading zeros (zeros occurring before non-zero digits) are not considered significant. For example, in the measurement 0.053 g, there are only two significant figures.
- Trailing zeros: Trailing zeros (zeros occurring after non-zero digits) are significant if there is a decimal point present. For example, in the measurement 2.00 L, there are three significant figures.
- Trailing zeros: Trailing zeros (zeros occurring after non-zero digits) are not significant if there is no decimal point present. For example, in the measurement 500 m, there is only one significant figure.
- Exact numbers: Exact numbers, such as counting or defined constants, are considered to have an infinite number of significant figures. For example, in the measurement 12 eggs, there are an infinite number of significant figures.
By following these rules, one can determine the number of significant figures in a measurement and accurately represent the precision and reliability of the measured value. It is important to correctly identify and use significant figures in calculations and when reporting scientific data.
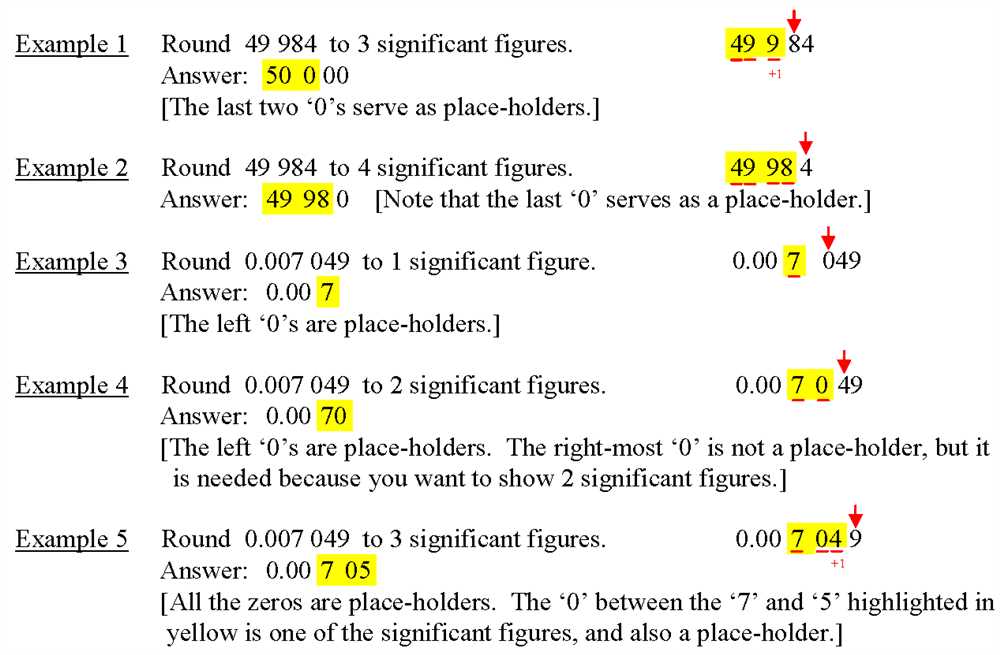
Rules for rounding off numbers
When working with numbers, it is often necessary to round off values to a specific number of significant figures or decimal places. Rounding off numbers helps to simplify calculations and make the results more readable and manageable. There are several rules that define how to properly round off numbers.
Rule 1: If the digit to be dropped is less than 5, then the preceding digit stays the same. For example, if we have the number 3.1415 and we want to round it off to three decimal places, we will get 3.142, because the digit 5 is greater than 5.
Rule 2: If the digit to be dropped is 5 or greater, then the preceding digit is increased by 1. For example, if we have the number 2.345 and we want to round it off to two decimal places, we will get 2.35, because the digit 5 is equal to 5 and we round up.
Rule 3: If the digit to be dropped is exactly 5 and there are no digits after it or all the digits after it are zeros, then the preceding digit is rounded up to an even number. This is known as the “round half to even” rule. For example, if we have the number 4.500 and we want to round it off to three decimal places, we will get 4.500, because the digit 5 is exactly 5 and all the digits after it are zeros.
To summarize, rounding off numbers involves making decisions based on the digit to be dropped and the digits that follow it. By following the rules mentioned above, we can ensure that our rounded off numbers are accurate and reflect the desired level of precision.
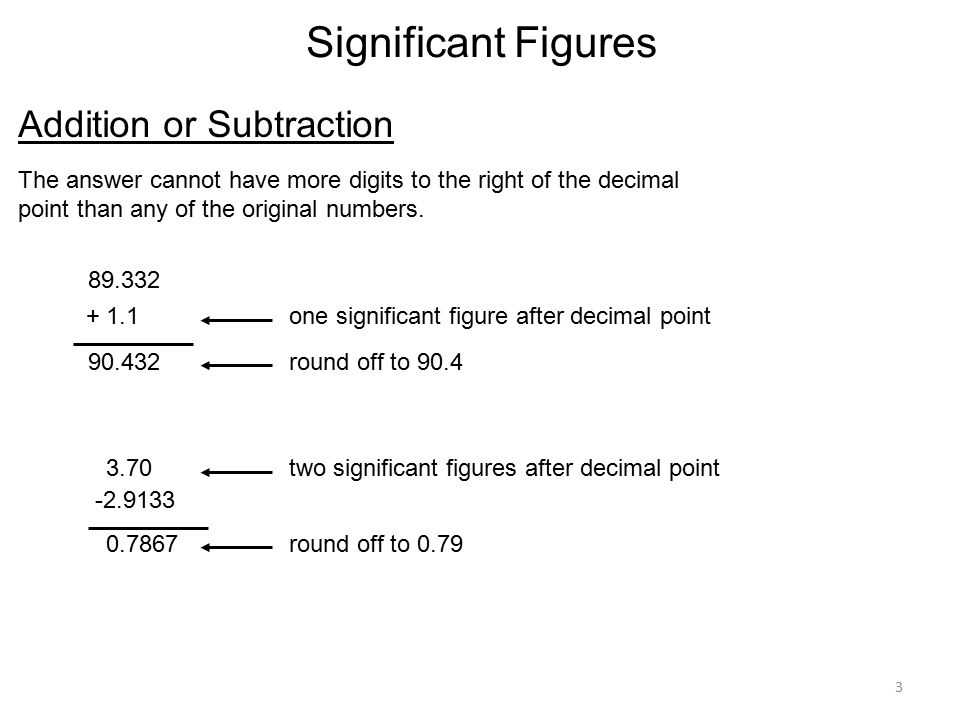
How to Perform Addition and Subtraction with Significant Figures
When performing addition or subtraction with significant figures, it is important to consider the number of decimal places in each term and align them accordingly. Here are the steps to follow:
Step 1: Align the decimal points of the numbers being added or subtracted.
Step 2: Count the number of significant figures to the right of the decimal point in each number. Choose the number with the fewest decimal places as your final answer’s decimal places. This ensures that your answer has the appropriate precision.
Step 3: Perform the addition or subtraction operation as usual, ignoring the decimal places for now.
Step 4: Round the final answer to the decimal place determined in Step 2. If the digit after the determined decimal place is less than 5, simply drop the remaining digits. If the digit after the determined decimal place is 5 or greater, round up by adding 1 to the determined decimal place.
For example, let’s consider the following addition problem: 13.625 + 7.13.
- Step 1: Align the decimal points as follows:
- Step 2: The number 13.625 has three decimal places, while 7.13 has two decimal places. Therefore, the final answer should have two decimal places.
- Step 3: Perform the addition: 13.625 + 7.13 = 20.755.
- Step 4: Round the final answer to two decimal places: 20.755 rounds to 20.76 (since the digit after the determined decimal place, 5, is greater than 5).
| 13.625 |
| + 7.13 |
| ——- |
By following these steps, you can ensure that your addition and subtraction results have the correct number of significant figures and appropriate precision.
How to perform multiplication and division with significant figures?

Performing multiplication and division with significant figures requires understanding how significant figures are determined in a given set of numbers and applying the rules accordingly.
Multiplication:
When multiplying numbers, the rule for significant figures is to count the number of significant figures in each number being multiplied together and use the smallest number of significant figures in the final answer.
For example, if you are multiplying 3.45 by 2.1, both numbers have three significant figures. Therefore, the multiplication should be carried out as follows:
- 3.45 x 2.1 = 7.245
However, since the number with the least number of significant figures is 2.1 (which has two significant figures), the final answer should be rounded to two significant figures, resulting in:
- 7.245 becomes 7.2 (rounded to two significant figures)
Division:
When dividing numbers, the rule for significant figures is similar to multiplication. Count the number of significant figures in each number involved in the division and use the smallest number of significant figures in the final answer.
For example, if you are dividing 5.46 by 0.23, the division is carried out as follows:
- 5.46 ÷ 0.23 = 23.7391304348
Since 0.23 has two significant figures, the final answer should be rounded to two significant figures:
- 23.7391304348 becomes 24 (rounded to two significant figures)
It is important to follow these rules when performing multiplication and division with significant figures to ensure the final answer reflects the appropriate level of precision based on the original numbers.
How to use scientific notation with significant figures?
Scientific notation is a way of expressing numbers that are very large or very small in a more concise and readable form. It is commonly used in scientific and mathematical calculations, as it allows for easier manipulation of numbers with a large range of values. When using scientific notation, it is important to consider the number of significant figures in the original measurement or calculation.
In scientific notation, a number is written in the form of A × 10ⁿ, where A is a number between 1 and 10 (including decimal fractions) and n is an integer. The value of n determines the magnitude of the number, while A represents the digits with significant figures. For example, the number 0.0032 can be written in scientific notation as 3.2 × 10⁻³, where A is 3.2 and n is -3.
When using scientific notation with significant figures, the value of A should be rounded to match the number of significant figures in the original measurement or calculation. For example, if the original measurement had three significant figures, A should be rounded to three significant figures as well. This ensures that the level of precision is maintained throughout calculations.
Additionally, when performing calculations with numbers in scientific notation, it is important to consider the rules of significant figures. In multiplication or division, the result should be rounded to the same number of significant figures as the least precise measurement or calculation. In addition, in addition or subtraction, the result should be rounded to the same number of decimal places as the least precise measurement or calculation.
In summary, when using scientific notation with significant figures, it is important to carefully round the value of A to match the number of significant figures in the original measurement or calculation. This maintains the level of precision and ensures accurate calculations. Additionally, the rules of significant figures should be followed when performing calculations with numbers in scientific notation.
Examples of calculations involving significant figures
Significant figures play a crucial role in scientific calculations as they ensure that the final result is accurate and reliable. Here are some examples of calculations involving significant figures:
Addition and subtraction:
When adding or subtracting numbers, the result should have the same number of decimal places as the least precise number in the calculation. For example, if we add 5.3 and 2.47, the result should be rounded to the nearest hundredth, which gives us 7.77.
Multiplication and division:
When multiplying or dividing numbers, the result should have the same number of significant figures as the least precise number in the calculation. For example, if we multiply 3.2 by 1.57, the result should be rounded to two significant figures, giving us 5.0. Similarly, if we divide 15.6 by 4.2, the result should be rounded to the nearest whole number, giving us 4.
Combining different operations:
When performing calculations that involve multiple operations, it is important to consider significant figures at each step. For example, if we have the expression (4.32 + 1.28) × 3.17, we first add 4.32 and 1.28, which gives us 5.6. Then, we multiply 5.6 by 3.17, giving us a final result of 17.8. The result should be rounded to two significant figures since the least precise number, 3.17, has two significant figures.
In summary, understanding significant figures is essential for accurate and reliable calculations in scientific and mathematical contexts. By following the rules of significant figures, we can ensure that our results are precise and reflect the limitations of the measurements used.