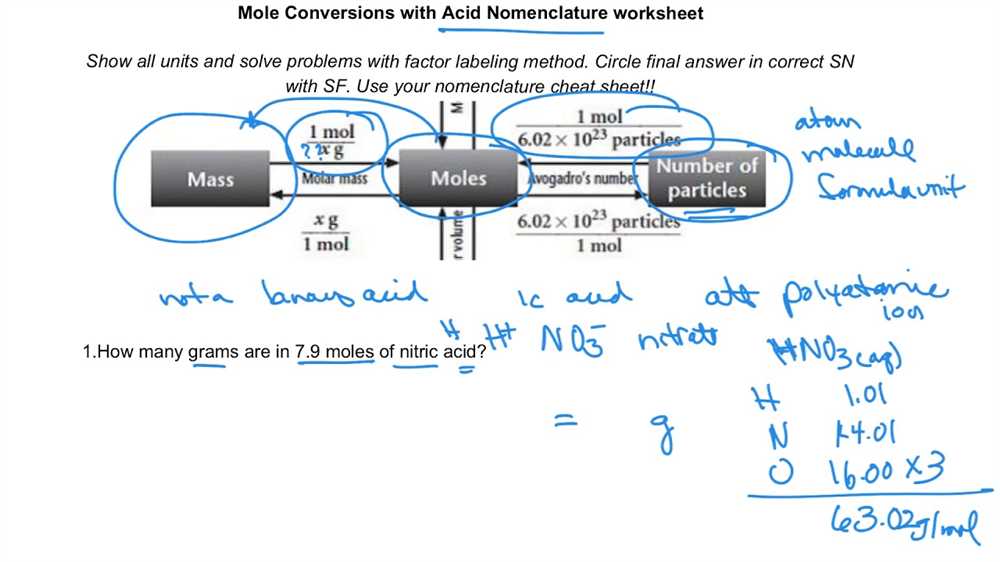
Understanding mole conversion is essential for students studying chemistry. Mole conversion is the process of converting between mass, moles, and particles of a substance. It involves using the molar mass, Avogadro’s number, and mathematical formulas to make conversions and solve problems.
This article serves as a comprehensive guide to mole conversion, providing a mole conversion worksheet and answers to help students practice and reinforce their understanding of this fundamental concept. The worksheet includes various conversion problems, such as converting grams to moles, moles to particles, and particles to grams.
By working through the mole conversion worksheet and checking their answers, students can solidify their understanding of the relationship between mass, moles, and particles. Through practice, they will become more proficient in performing mole conversions and applying them to real-world scenarios.
Mole Conversion Worksheet and Answers
Understanding mole conversion is crucial in chemistry as it allows us to convert between different units of measurement, specifically regarding atoms and molecules. A mole is a unit that represents the amount of a substance, and it is equal to Avogadro’s number, which is approximately 6.022 × 10^23. In mole conversion, we use this number to convert between different units, such as grams, molecules, and atoms.
The mole conversion worksheet provides practice problems to reinforce this concept. It typically includes a series of questions where the student is given a certain amount of a substance in one unit and is asked to convert it to another unit. The worksheet may also involve balancing chemical equations and determining the correct stoichiometric ratios for mole conversions.
Here are some sample problems from a mole conversion worksheet with their corresponding answers:
- 1. How many moles are in 10 grams of oxygen? Answer: 0.312 moles
- 2. Convert 2.5 moles of sodium chloride to grams. Answer: 117.88 grams
- 3. What is the molar mass of water (H2O)? Answer: 18.015 moles
- 4. How many molecules are in 5 moles of carbon dioxide (CO2)? Answer: 3.011 × 10^24 molecules
By practicing these mole conversion problems and understanding the correct steps and calculations, students can improve their comprehension and skills in working with moles and conversions. It is important to remember the conversion factors and relationships between different units to accurately convert between them.
Overall, a mole conversion worksheet provides valuable practice and reinforcement for students studying chemistry, helping them develop a solid understanding of moles and their conversions.
What is a Mole?

Mole is a fundamental concept in chemistry that helps in counting and measuring quantities of substances on the atomic and molecular scale. It is a unit of measurement that allows chemists to convert between different types of particles, such as atoms, molecules, or ions.
A mole represents a specific number of particles, similar to how a dozen represents 12 items. In the case of a mole, that specific number is known as Avogadro’s number, which is approximately 6.022 x 10^23. This means that one mole of any substance contains 6.022 x 10^23 particles, whether they are atoms, molecules, or ions.
How is the concept of a mole used?
The concept of a mole is used in a variety of ways in chemistry. It helps determine the amount of a substance needed for a chemical reaction, as well as the amount of product that can be produced. It is also used to calculate the molar mass of a substance, which is the mass in grams of one mole of that substance.
Chemists use the mole to convert between different units of measurement, such as grams to moles or moles to particles. This conversion is done using molar mass, which is the mass of one mole of a substance. By knowing the molar mass of a substance, chemists can calculate the number of moles present in a given mass of that substance.
The concept of a mole is vital for understanding and performing calculations in chemistry. It allows chemists to work with individual particles on a larger scale, making it easier to measure and manipulate substances in the laboratory.
In conclusion, the mole is a fundamental concept in chemistry that represents a specific number of particles. It is used to measure and convert between different units of measurement and is essential for performing calculations and understanding the atomic and molecular scale.
Avogadro’s Number and Molar Mass
The concept of Avogadro’s number and molar mass play a crucial role in understanding the relationship between atoms, molecules, and moles. Avogadro’s number, denoted as NA, is the number of atoms or molecules present in one mole of a substance. It is approximately equal to 6.022 x 10^23. This number allows chemists to convert between mass and the number of particles.
To understand the significance of Avogadro’s number, it’s essential to comprehend molar mass. The molar mass of a substance is the mass of one mole of that substance and is expressed in grams per mole (g/mol). It is calculated by summing up the atomic masses of all the atoms in the molecule. The molar mass is also known as the molecular weight or molecular mass.
Avogadro’s number and molar mass are interconnected. It allows chemists to convert between grams and moles of a substance using the following equation:
moles = mass (g) / molar mass (g/mol)
Similarly, the number of atoms or molecules can be determined using the equation:
number of particles = moles x Avogadro’s number
In chemistry, these conversions are essential for various calculations, such as determining the amount of reactants or products in a chemical reaction, calculating the amount of substance needed for a given reaction, or understanding the composition of a compound.
- Avogadro’s number: Approximately 6.022 x 10^23 atoms or molecules per mole.
- Molar mass: The mass of one mole of a substance, expressed in grams per mole (g/mol).
- Conversions: Grams to moles and moles to number of particles.
Understanding Avogadro’s number and molar mass is fundamental in stoichiometry and the quantitative study of chemical reactions. These concepts provide a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and moles, allowing chemists to quantify chemical phenomena.
Converting Moles to Grams
Converting moles to grams is an essential skill in chemistry. It allows us to determine the mass of a substance based on its chemical formula and the number of moles present. This conversion is particularly important when dealing with chemical reactions and stoichiometry, where understanding the mass of reactants and products is crucial.
To convert moles to grams, we need to use the molar mass of the substance. The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). It can be calculated by summing the atomic masses of all the atoms in the chemical formula.
Example: Let’s say we have 2 moles of carbon dioxide (CO2). To convert moles to grams, we need to know the molar mass of carbon dioxide. The molar mass of carbon (C) is 12.01 g/mol, and the molar mass of oxygen (O) is 16.00 g/mol. Adding these masses together, we get a molar mass of 44.01 g/mol for carbon dioxide. To convert the 2 moles of carbon dioxide to grams, we multiply by the molar mass: 2 mol * 44.01 g/mol = 88.02 grams.
When converting moles to grams, it is important to use the correct molar mass and pay attention to the units. This conversion allows us to relate the quantitative aspects of chemical reactions to the real-world mass of substances involved.
Converting Grams to Moles
Converting grams to moles is an essential skill in chemistry as it allows us to understand the relationship between the mass of a substance and the number of particles it contains. The conversion is based on the molar mass of the substance, which is the mass of one mole of that substance.
To convert grams to moles, we use the formula:
Moles = grams / molar mass
First, we need to determine the molar mass of the substance by adding up the atomic masses of all the elements present in the compound. The atomic masses can be found on the periodic table. Once we have the molar mass, we simply divide the given mass in grams by the molar mass to obtain the number of moles.
Let’s take an example to illustrate this concept. Suppose we have 25 grams of water (H₂O) and want to convert it to moles. The molar mass of water is approximately 18 grams/mol. Using the formula, we can calculate:
Moles = 25g / 18g/mol = 1.39 mol
Therefore, 25 grams of water is equivalent to 1.39 moles.
It is important to note that the conversion from grams to moles is not limited to simple compounds like water. It can be applied to any substance, including complex molecules and ions. By converting grams to moles, we can accurately measure and compare the amounts of different substances in chemical reactions and understand their stoichiometry.
Converting Moles to Particles
Converting moles to particles is an essential skill in chemistry. It allows us to determine the number of particles, such as atoms, molecules, or ions, in a given amount of substance. The conversion is based on Avogadro’s number, which states that one mole of any substance contains exactly 6.022 × 10^23 particles.
To convert moles to particles, you need to multiply the number of moles by Avogadro’s number. The formula is:
Number of particles = Number of moles × Avogadro’s number
For example, let’s say you have 2 moles of oxygen atoms. To determine the number of oxygen atoms, you would use the formula:
Number of oxygen atoms = 2 moles × (6.022 × 10^23 atoms/mole)
This calculation would give you the total number of oxygen atoms in the given sample. It is important to note that Avogadro’s number is a constant, and it does not change regardless of the substance being considered.
Converting moles to particles allows chemists to make precise calculations and understand the relationships between different substances in a chemical reaction. It is a fundamental concept in stoichiometry, which deals with the quantitative relationships of substances in chemical reactions. Mastering this skill is crucial for anyone studying chemistry or working in the field.
Converting Particles to Moles
When working with chemical reactions and stoichiometry, it is often necessary to convert the number of particles of a substance into moles. This conversion allows us to compare different substances and determine the amounts needed for a reaction.
To convert particles to moles, we use Avogadro’s number, which states that one mole of any substance contains 6.022 x 10^23 particles. This constant allows us to establish a relationship between the number of particles and the amount in moles.
To perform the conversion, we simply divide the given number of particles by Avogadro’s number. This calculation gives us the amount of substance in moles. It is important to remember to write the units correctly, as moles should always be written as “mol”.
For example, if we have 1.204 x 10^23 particles of sodium chloride (NaCl), we can convert this amount to moles by dividing it by Avogadro’s number:
1.204 x 10^23 particles NaCl / (6.022 x 10^23 particles/mol) = 0.2 mol NaCl
Therefore, 1.204 x 10^23 particles of sodium chloride is equal to 0.2 moles of sodium chloride.
Converting particles to moles is an essential skill in chemistry, as it allows us to accurately calculate the amounts of substances involved in a reaction. By understanding this conversion, we can analyze and interpret chemical reactions more effectively.
Practice Problems
Here are some practice problems to help you master mole conversions.
Problem 1:

A sample of sodium chloride (NaCl) contains 2.34 moles. How many grams of sodium chloride is this?
To solve this problem, we can use the molar mass of NaCl, which is 58.44 grams/mol. We can set up a conversion factor with moles on one side and grams on the other:
2.34 mol NaCl × 58.44 g NaCl/mol = 136.2896 g NaCl
Problem 2:
A sample of glucose (C6H12O6) has a mass of 25 grams. How many moles of glucose are in this sample?
To solve this problem, we need to determine the molar mass of glucose, which is 180.18 grams/mol. We can set up a conversion factor with grams on one side and moles on the other:
25 g glucose × 1 mol glucose/180.18 g glucose = 0.13855 mol glucose
Problem 3:
A sample of water (H2O) contains 0.5 moles. How many molecules of water are in this sample?
To solve this problem, we need to use Avogadro’s number, which is 6.022 × 10^23 molecules/mol. We can set up a conversion factor with moles on one side and molecules on the other:
0.5 mol H2O × 6.022 × 10^23 molecules H2O/mol = 3.011 × 10^23 molecules H2O
These practice problems will help you develop your skills in converting between moles and grams, as well as moles and molecules. Remember to always use the correct molar mass or Avogadro’s number when setting up your conversion factors.