
Energy and Its Forms: In this section, we will explore the concept of energy and its various forms. Energy is defined as the ability to do work or cause change. It exists in different forms, such as kinetic energy, potential energy, thermal energy, chemical energy, and electrical energy. Understanding these forms of energy is crucial in comprehending how energy is transferred and transformed in various systems.
Kinetic Energy: Kinetic energy refers to the energy possessed by an object due to its motion. The formula to calculate kinetic energy is ½mv^2, where m represents the mass of the object and v represents its velocity. The faster an object moves or the greater its mass, the more kinetic energy it possesses. Examples of kinetic energy include a rolling ball, a speeding car, or a swinging pendulum.
Potential Energy: Potential energy is the energy stored in an object that can be released to do work. It can be classified into several types, including gravitational potential energy, elastic potential energy, and chemical potential energy. Gravitational potential energy is related to an object’s height and its weight, while elastic potential energy is associated with objects that can be stretched or compressed. Chemical potential energy is stored in chemical bonds and can be released through reactions. Understanding potential energy is crucial in understanding systems like a roller coaster or a stretched spring.
Thermal Energy: Thermal energy, also known as heat energy, is the energy produced by the random motion of particles in a substance. It is directly related to the temperature of an object or substance. The higher the temperature, the greater the thermal energy. Thermal energy can be transferred through conduction, convection, or radiation. This form of energy is essential in understanding concepts like heat transfer, energy conservation, and thermodynamics.
Chemical Energy: Chemical energy refers to the energy stored in the bonds between atoms and molecules. It is released or absorbed during chemical reactions. Examples of chemical energy include the energy stored in food, fuels, or batteries. Understanding chemical energy is crucial in various fields, such as biology, chemistry, and environmental science.
Electrical Energy: Electrical energy is the energy associated with the movement of electric charges. It can come from various sources, such as batteries, power plants, or renewable energy sources like solar panels or wind turbines. Understanding electrical energy is vital in comprehending concepts like circuits, electricity consumption, and electrical power generation.
In conclusion, energy exists in various forms, and understanding these forms is vital in comprehending how energy is transferred and transformed. The different forms of energy discussed in this section include kinetic energy, potential energy, thermal energy, chemical energy, and electrical energy. Each form has its unique characteristics and is relevant in different fields of science and everyday life. By studying these forms, we can better understand the fundamental principles and processes of energy in our world.
Section 15.1 Energy and Its Forms Answer Key
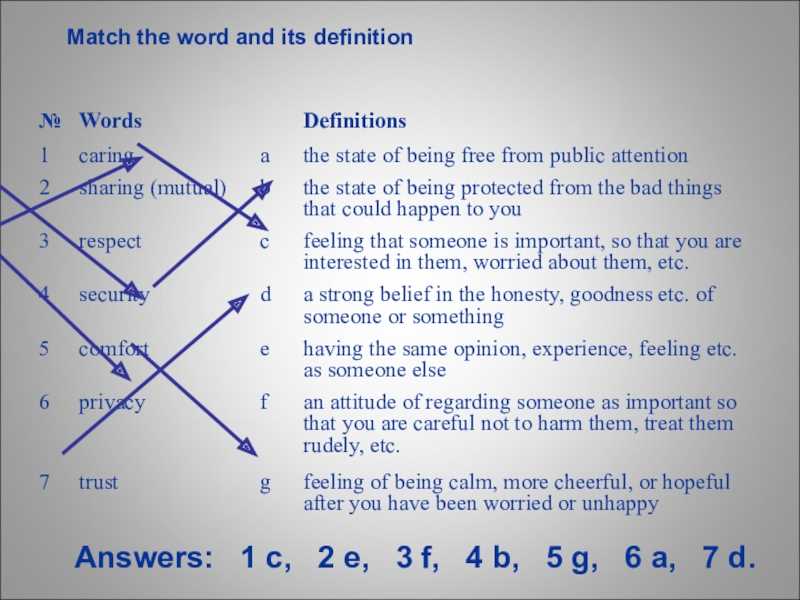
In this section, we will explore the concept of energy and its various forms. Energy is the ability to do work or cause change. It is a fundamental concept in physics and is present in all aspects of our daily lives. The answer key provided here will help you understand and identify different forms of energy.
1. Mechanical Energy: This form of energy is associated with the movement or position of an object. It includes both kinetic energy, which is the energy of motion, and potential energy, which is the energy stored in an object due to its position or condition. Examples of mechanical energy include a moving car or a stretched rubber band.
2. Thermal Energy: Thermal energy is the internal energy of an object due to the motion of its particles. It is commonly referred to as heat energy and is transferred between objects through conduction, convection, or radiation. It is responsible for changes in temperature and can be felt as hot or cold. Examples of thermal energy include a boiling pot of water or a warm cup of coffee.
3. Chemical Energy: Chemical energy is stored in the bonds between atoms and molecules. It is released or absorbed during a chemical reaction. Examples of chemical energy include the energy stored in fossil fuels, such as gasoline, or the energy released during the digestion of food in our bodies.
4. Electrical Energy: Electrical energy is the energy associated with the movement of electric charges. It is generated by the flow of electrons through conductors, such as wires, and is used to power various devices and systems. Examples of electrical energy include the energy provided by a battery or the electricity used to power a lightbulb.
5. Nuclear Energy: Nuclear energy is the energy stored in the nucleus of an atom. It is released during nuclear reactions, such as nuclear fission or fusion. Nuclear power plants use this energy to generate electricity. Examples of nuclear energy include the energy released by the sun or the energy produced in a nuclear reactor.
In conclusion, energy exists in various forms and is essential for all processes and phenomena in the universe. Understanding the different forms of energy allows us to better comprehend and harness its power for the benefit of society.
What is Energy?
Energy is a crucial concept in physics, as it is the ability to do work or cause change. It is a fundamental property of matter and is present in various forms. Understanding energy is essential for comprehending the behavior of the universe.
Forms of Energy:
- Kinetic Energy: This is the energy possessed by an object due to its motion. The faster an object moves, the more kinetic energy it possesses.
- Potential Energy: This is the energy possessed by an object due to its position or state. It can be stored energy that can be transformed into other forms of energy.
- Thermal Energy: This is the energy associated with the temperature of an object. It is a form of kinetic energy that exists at the molecular level and is responsible for heating and cooling.
- Chemical Energy: This is the potential energy stored in the bonds between atoms and molecules. It is released or absorbed when chemical reactions occur.
- Electrical Energy: This is the energy associated with the flow of electric charges. It is commonly used for powering electronic devices and is generated from various sources such as batteries or power plants.
- Nuclear Energy: This is the energy released during nuclear reactions, such as fission (splitting of atomic nuclei) or fusion (combining of atomic nuclei). It is incredibly powerful and is the source of energy for the sun.
- Light Energy: This is the energy carried by electromagnetic waves. It is responsible for our ability to see and is utilized in various technologies such as lasers and solar panels.
- Sound Energy: This is the energy carried by sound waves. It is produced by the vibration of particles in a medium and is used for communication and detection.
Energy can be converted from one form to another, but it cannot be created or destroyed, according to the law of conservation of energy. This principle is fundamental to understanding the behavior of energy in various systems and processes.
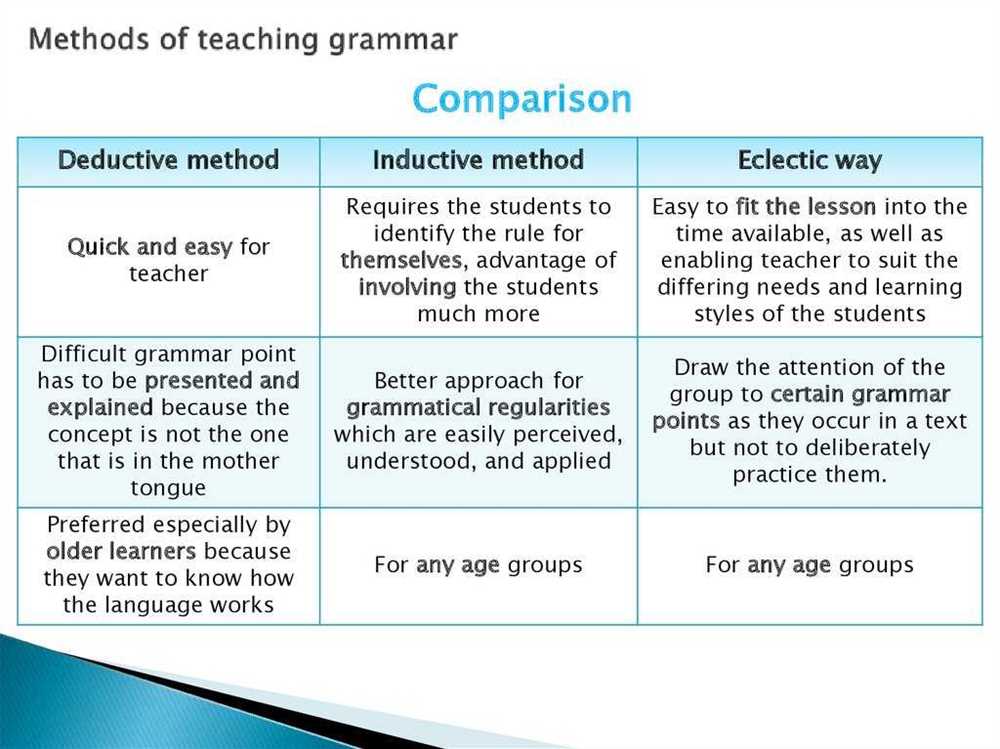
The Laws of Thermodynamics

The Laws of Thermodynamics are fundamental principles that govern the behavior of energy in physical systems. These laws provide a framework for understanding how energy can be transformed from one form to another, and how it flows within a system.
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transferred or converted from one form to another. This law is also known as the law of conservation of energy. It implies that the total energy of a closed system remains constant over time.
The Second Law of Thermodynamics states that the total entropy, or disorder, of a closed system always increases over time. Entropy is a measure of the amount of energy that is unavailable to do work. This law implies that natural processes tend to move towards a state of greater disorder.
The Third Law of Thermodynamics states that as the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value. This law sets a limit on the lowest temperature attainable in nature and provides a basis for understanding phenomena such as superconductivity and superfluidity.
The laws of thermodynamics have wide-ranging applications in various fields such as physics, chemistry, and engineering. These principles are used to analyze and design energy systems, understand the behavior of materials at different temperatures, and predict the outcomes of chemical reactions. They are also essential for understanding concepts such as heat transfer, work, and energy efficiency.
By understanding and applying the laws of thermodynamics, scientists and engineers can develop more efficient and sustainable energy technologies, improve the performance of existing systems, and explore new frontiers in science and engineering.
Forms of Energy
Energy is the ability to do work or cause a change. It exists in various forms and can be transferred from one object to another. Understanding the different forms of energy is crucial in understanding how the world around us operates.
1. Kinetic Energy: Kinetic energy is the energy of motion. Any object that is moving has kinetic energy. The amount of kinetic energy an object possesses depends on its mass and velocity. For example, a moving car and a flying bird both possess kinetic energy.
2. Potential Energy: Potential energy is the energy an object possesses due to its position or condition. It can be thought of as stored energy, waiting to be released. Some examples of potential energy include a stretched rubber band, a book on a shelf, or water behind a dam. When released, this energy can be converted into other forms.
3. Thermal Energy: Thermal energy is the energy associated with the temperature of objects. It is a type of kinetic energy that results from the movement and vibration of particles at the atomic or molecular level. As objects heat up, their thermal energy increases. Examples of thermal energy include the heat from a fire, the warmth of the sun, or the steam in a kettle.
4. Chemical Energy: Chemical energy is energy stored in the bonds of atoms and molecules. It is released during chemical reactions. Food is a prime example of chemical energy, as our bodies convert the energy stored in food molecules into forms that can be used to fuel our daily activities.
5. Electrical Energy: Electrical energy is the energy associated with moving electric charges, such as those found in wires and batteries. It is a common form of energy used to power devices and appliances in our daily lives.
6. Sound Energy: Sound energy is the energy produced by vibrations that can be heard by the human ear. When an object vibrates, it creates sound waves that travel through a medium, such as air or water. Examples of sound energy include music, the sound of a bell ringing, or the noise of a car engine.
7. Light Energy: Light energy is a form of electromagnetic radiation that can be detected by the human eye. It travels in the form of waves and carries energy. Sunlight, a lamp, and a candle flame all emit light energy.
8. Nuclear Energy: Nuclear energy is the energy stored in the nucleus of an atom. It is released during nuclear reactions, such as those that occur in the sun or in nuclear power plants. Nuclear energy is highly concentrated and can generate large amounts of power.
These are just a few examples of the different forms of energy that exist. Energy cannot be created or destroyed but only transferred or transformed from one form to another. Understanding how energy works and its various forms is essential in many fields of science and engineering.
Potential Energy

Potential energy is the stored energy an object possesses due to its position, shape, or condition. It is the energy that an object has the potential to release and convert into other forms of energy. There are various types of potential energy, including gravitational potential energy, elastic potential energy, chemical potential energy, and nuclear potential energy.
Gravitational potential energy is the energy an object possesses due to its position in a gravitational field. The higher an object is lifted, the more potential energy it has. This type of potential energy can be calculated using the equation PE = mgh, where PE is the potential energy, m is the mass of the object, g is the acceleration due to gravity, and h is the height of the object.
Elastic potential energy is the energy stored in objects that can be compressed or stretched, such as a spring or a rubber band. When an object is compressed or stretched, it stores potential energy that can be released when the object returns to its original shape. The amount of elastic potential energy depends on the amount of deformation and the spring constant of the material.
Chemical potential energy is the energy stored in the chemical bonds of molecules. It is released when chemical reactions occur. For example, when fuel is burned, the chemical potential energy in the fuel is converted into thermal energy, which is used to perform work.
Nuclear potential energy is the energy stored in the nucleus of an atom. It is released during nuclear reactions, such as nuclear fusion or fission. Nuclear reactions involve changes in the arrangement of protons and neutrons in the nucleus, releasing a large amount of energy.
Overall, potential energy is an important concept in physics and is crucial in understanding how energy is stored and converted in various systems.
Kinetic Energy
Kinetic energy is the energy of an object due to its motion. It is defined as the work needed to accelerate the object from rest to its current velocity. This means that an object with a greater mass or a greater velocity will have a greater kinetic energy. Kinetic energy can be calculated using the formula:
KE = 1/2 mv^2
Where KE is the kinetic energy, m is the mass of the object, and v is the velocity of the object. The unit of kinetic energy is Joules (J).
For example, consider a car moving at a velocity of 20 meters per second (m/s), with a mass of 1000 kilograms (kg). Using the formula above, we can calculate the kinetic energy of the car:
- KE = 1/2 * 1000 kg * (20 m/s)^2
- KE = 1/2 * 1000 kg * 400 m^2/s^2
- KE = 200,000 J
Therefore, the car has a kinetic energy of 200,000 Joules.
Kinetic energy is an important concept in physics and is used to describe the energy of moving objects. It is an essential component of many everyday phenomena, such as the movement of vehicles, the flight of airplanes, and the motions of everyday objects.