
Hexamethylenetetramine, also known as methenamine, is a white crystalline powder substance that is commonly used as a reagent in organic synthesis. It is a stable and versatile compound that is widely employed in various industries, including pharmaceuticals, agriculture, and chemical manufacturing. This article provides essential information about the material safety data sheet (MSDS) for hexamethylenetetramine, which outlines important safety measures and precautions for handling this chemical.
The MSDS for hexamethylenetetramine serves as a comprehensive guide for individuals working with this compound. It provides detailed information regarding its physical and chemical properties, potential hazards, and recommended safety practices. The safety data sheet includes crucial details such as the substance’s flammability, toxicity levels, and recommended storage and disposal methods.
One of the primary purposes of an MSDS is to ensure the safety of individuals working with hexamethylenetetramine or coming into contact with it. The document highlights the necessary personal protective equipment (PPE) that should be worn when handling the substance, such as goggles, gloves, and protective clothing. It also offers guidance on how to respond to accidents or incidents involving hexamethylenetetramine, such as spills or exposure to the skin or eyes.
What is Hexamethylenetetramine?
Hexamethylenetetramine, also known as methenamine, is a chemical compound that is commonly used in various industrial applications. It is a white crystalline powder with a molecular formula of C6H12N4. Hexamethylenetetramine is highly soluble in water and has a characteristic odor.
Hexamethylenetetramine is primarily used as a starting material for the production of a variety of chemicals, including pharmaceuticals, resins, and plastics. It is often used as a curing agent in epoxy resins and as a catalyst in the synthesis of various organic compounds. In addition, it is used as a fuel additive to improve the combustion efficiency of gasoline and as a corrosion inhibitor in cooling systems.
When handling hexamethylenetetramine, it is important to take proper safety precautions. It may be harmful if swallowed, inhaled, or absorbed through the skin. It can cause irritation to the eyes, skin, and respiratory system. It is recommended to wear protective clothing, gloves, and goggles when working with this compound. In case of accidental exposure, it is important to seek medical attention immediately.
To ensure safe handling and storage of hexamethylenetetramine, it is necessary to follow the guidelines provided in its Material Safety Data Sheet (MSDS). The MSDS contains detailed information on the chemical’s physical and chemical properties, potential hazards, first aid measures, and proper handling and storage procedures. It is important to read and understand the MSDS before working with hexamethylenetetramine to ensure the safety of both humans and the environment.
Definition and chemical properties
Hexamethylenetetramine, also known as methenamine, is a white crystalline compound with the chemical formula C6H12N4. It is a solid at room temperature and has a distinctive odor. Hexamethylenetetramine is highly soluble in water and forms a clear and colorless solution.
The compound is formed by the condensation reaction of formaldehyde and ammonia. It is commonly used as a starting material for the synthesis of various organic compounds, such as resins, rubber accelerators, pharmaceuticals, and fuel to name a few. Hexamethylenetetramine is also used as a stabilizer for formaldehyde-based resins, as a preservative in cosmetics, and as a corrosion inhibitor in certain industrial processes.
Chemical properties:
- Molecular weight: 140.19 g/mol
- Melting point: 280-300°C
- Boiling point: Decomposes above 282°C
- Solubility: Highly soluble in water
- Density: 1.33 g/cm³
- Flash point: No data available
- pH: No data available
Overall, hexamethylenetetramine is a versatile compound used in various industrial applications due to its unique chemical properties and reactivity.
Uses and Applications
Hexamethylenetetramine, also known as hexamine or methenamine, is a versatile compound that has various uses and applications in different industries.
One of the most common uses of hexamethylenetetramine is as a key ingredient in certain types of resins and plastics. It is often used as a crosslinking agent in these materials, helping to improve their strength and durability. Hexamethylenetetramine can be found in products such as bakelite, a type of heat-resistant and electrically insulating plastic.
In addition to its role in the manufacturing of plastics, hexamethylenetetramine has applications in the pharmaceutical industry. It is used as a precursor in the synthesis of various medications, including antibiotics and antiseptics. The compound’s unique chemical properties make it a useful building block for the creation of these important drugs.
Hexamethylenetetramine also finds applications in the field of photography. It is used as a developing agent in black and white photography, helping to bring out the desired image on the photographic paper. The compound’s ability to react with silver compounds makes it a valuable tool in the darkroom.
Furthermore, hexamethylenetetramine has applications in the field of fuel technology. It can be added to gasoline as a fuel additive to improve its octane rating and prevent engine knocking. This compound helps to optimize the combustion process in the engine, resulting in better performance and efficiency.
In summary, hexamethylenetetramine is a versatile compound with diverse uses and applications. From its role in the manufacturing of plastics and pharmaceuticals to its contribution to the field of photography and fuel technology, hexamethylenetetramine plays a significant role in various industries, demonstrating its importance as a chemical compound.
Health and Safety Information
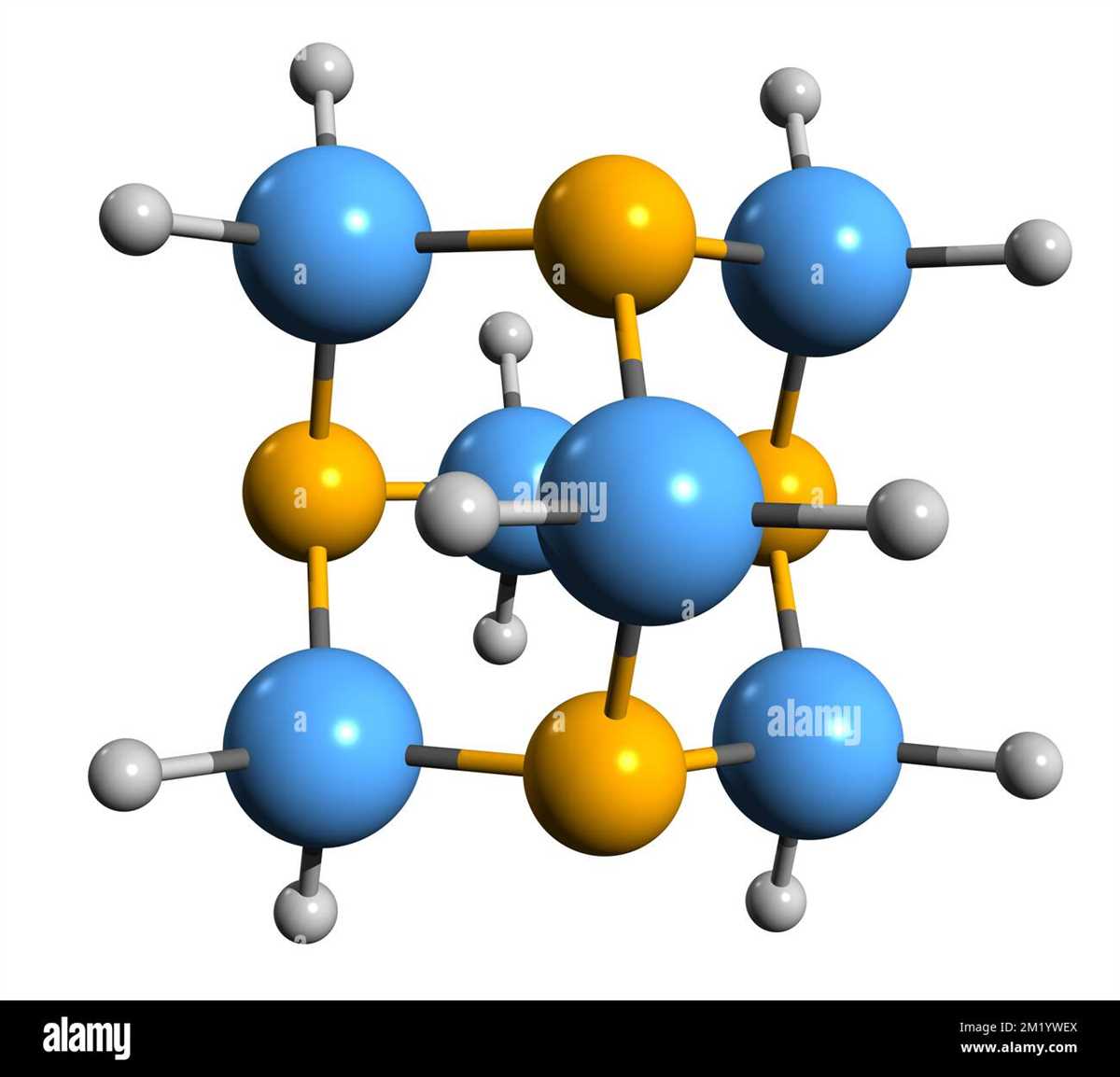
Hexamethylenetetramine is a chemical compound commonly used in various industrial applications. As with any chemical substance, it is important to handle hexamethylenetetramine with caution and adhere to proper health and safety measures.
Hazard identification:
- Hexamethylenetetramine is classified as a hazardous substance and may cause irritation to the respiratory system, skin, and eyes.
- Inhalation of hexamethylenetetramine dust or fumes may cause respiratory irritation, coughing, or difficulty breathing.
- Contact with the skin may cause redness, itching, or burning sensations.
- If hexamethylenetetramine comes into contact with the eyes, it may cause irritation, redness, or blurred vision.
- Ingestion of hexamethylenetetramine may result in gastrointestinal discomfort, nausea, or vomiting.
Preventive measures:
- When working with hexamethylenetetramine, it is crucial to wear appropriate personal protective equipment, including gloves, goggles, and a respiratory mask.
- Adequate ventilation should be ensured in the working area to prevent the buildup of hexamethylenetetramine vapors or dust.
- In case of skin contact, immediately wash the affected area with plenty of water and mild soap. If irritation persists, seek medical attention.
- If hexamethylenetetramine gets into the eyes, rinse them gently with water for several minutes while keeping the eyelids open. Seek medical attention if irritation continues.
- Ingestion of hexamethylenetetramine is unlikely in normal handling conditions. However, if accidentally swallowed, do not induce vomiting. Seek immediate medical attention and provide the medical professional with the substance’s safety data sheet.
- Proper storage and handling procedures should be followed to minimize the risk of accidents or contamination. Store hexamethylenetetramine in a cool, dry place away from heat, open flames, or other sources of ignition.
Emergency procedures:
In the event of a spill, containment measures should be taken to prevent the spread of the substance. Small spills can be absorbed with an inert material, such as sand or vermiculite. Larger spills may require professional assistance. Avoid direct contact with the spilled substance and use appropriate protective equipment.
First aid measures:
If someone experiences symptoms of exposure to hexamethylenetetramine, remove them from the contaminated area and seek immediate medical attention. Provide the healthcare professional with the material safety data sheet and inform them of the extent of exposure.
By following proper health and safety precautions, the risks associated with hexamethylenetetramine can be minimized, ensuring a safe working environment for all individuals involved.
Handling and storage guidelines
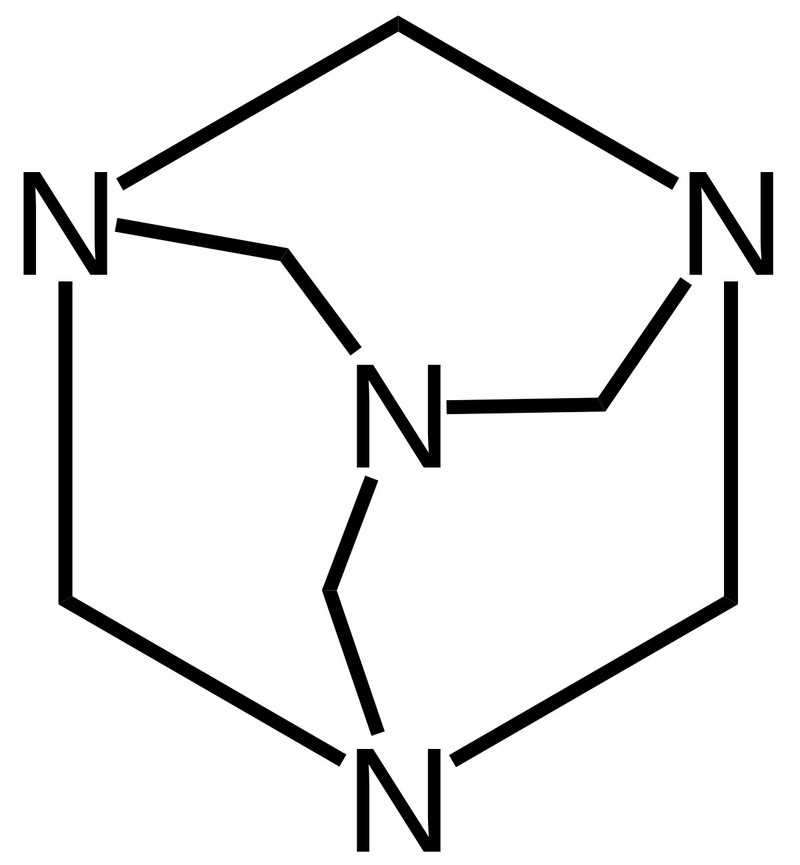
Handling:
- Wear appropriate protective equipment, including gloves, goggles, and lab coat, when handling hexamethylenetetramine.
- Handle the substance in a well-ventilated area to avoid inhalation of vapors or dust.
- Avoid contact with skin, eyes, and clothing. In case of contact, immediately rinse the affected area with plenty of water.
- Avoid ingestion. If swallowed, seek medical attention immediately.
- Do not eat, drink, or smoke while handling hexamethylenetetramine.
- Do not release the substance into the environment.
Storage:
- Store hexamethylenetetramine in a cool, dry, and well-ventilated area.
- Keep the substance in its original tightly closed container, away from incompatible materials.
- Avoid exposure to heat, sparks, and open flames.
- Store away from direct sunlight.
- Keep the substance out of reach of children and unauthorized personnel.
These guidelines are provided to ensure safe handling and storage of hexamethylenetetramine. Following these recommendations will help minimize the risks associated with this substance and maintain a safe working environment.
Environmental impacts and regulations

Hexamethylenetetramine is considered to have low to moderate toxicity to aquatic organisms. It has been found to be toxic to certain species of fish and invertebrates at high concentrations.
In terms of environmental regulations, hexamethylenetetramine is not classified as hazardous waste. However, it is still subject to regulations and guidelines set by various governing bodies.
- The Environmental Protection Agency (EPA) has set limits on the amount of hexamethylenetetramine that can be discharged into water bodies.
- The Occupational Safety and Health Administration (OSHA) has established workplace exposure limits for hexamethylenetetramine.
- The European Chemicals Agency (ECHA) has also classified hexamethylenetetramine as hazardous to aquatic life and has set limits on its use and release into the environment.
Overall, hexamethylenetetramine has the potential to have adverse effects on the environment if not properly regulated and managed. Therefore, it is important for industries and individuals to follow the guidelines and regulations set by relevant authorities to protect the environment.
Summary:
