
Chemical bonding is a fundamental concept in chemistry that explains how atoms are held together to form molecules and compounds. Understanding chemical bonding is crucial for understanding the properties and behavior of substances, as well as for predicting and explaining chemical reactions.
A chemical bonding test is a common assessment used by teachers to evaluate students’ understanding of the different types of chemical bonds, such as ionic, covalent, and metallic bonds. It tests their knowledge of bond formation, bond strength, and the properties associated with different types of bonds, among other concepts.
When grading a chemical bonding test, teachers often provide an answer key that contains the correct answers to each question. This allows students to compare their answers and identify any mistakes they may have made. The answer key also helps teachers determine each student’s level of understanding and provide targeted feedback.
While the answer key serves as a guide, it is important to note that chemistry is not always black and white. Some questions may have multiple correct answers or require students to apply their knowledge to real-life scenarios. In such cases, teachers may provide additional explanations or discuss possible alternate answers with their students.
Chemical Bonding Test Answer Key

In this chemical bonding test answer key, we will provide the correct answers for the questions that were asked in the test. It is important to note that understanding chemical bonding is crucial for understanding how different elements combine to form compounds.
Question 1:
What is chemical bonding?
Answer:
Chemical bonding refers to the process of joining atoms together to form a molecule or compound. It involves the sharing, gaining, or losing of electrons between atoms to achieve stability.
Question 2:
What are the different types of chemical bonding?
Answer:
The different types of chemical bonding are:
- Ionic bonding: It occurs when one or more electrons are transferred from one atom to another, resulting in the formation of ions.
- Covalent bonding: It occurs when electrons are shared between atoms. It can be either polar or nonpolar, depending on the electronegativity difference between the atoms involved.
- Metallic bonding: It occurs in metals, where delocalized electrons are shared among a lattice of positively charged metal ions.
Question 3:
What determines the type of chemical bonding?
Answer:
The type of chemical bonding is determined by the difference in electronegativity between the atoms involved. If the difference is large, ionic bonding occurs. If the difference is small, covalent bonding occurs. Metallic bonding occurs in metals due to the delocalized nature of electrons.
Question 4:
How are chemical bonds represented?
Answer:
Chemical bonds are represented using chemical formulas, Lewis structures, or molecular models. Chemical formulas provide the ratio of different atoms in a compound, Lewis structures show the arrangement of atoms and electrons, and molecular models provide a three-dimensional representation of the molecule.
Question 5:
What are some examples of compounds with different types of chemical bonding?
Answer:
Examples of compounds with different types of chemical bonding include:
- NaCl (sodium chloride): Ionic bonding
- H2O (water): Covalent bonding
- Fe (iron): Metallic bonding
It is important to review the correct answers provided in this chemical bonding test answer key to enhance understanding of chemical bonding concepts.
Overview of Chemical Bonding
Chemical bonding is the process by which atoms combine to form molecules and compounds. Atoms are held together by various forces of attraction, known as chemical bonds. These bonds are formed when valence electrons, the outermost electrons of an atom, are shared, transferred, or attracted to other atoms.
There are three main types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds.
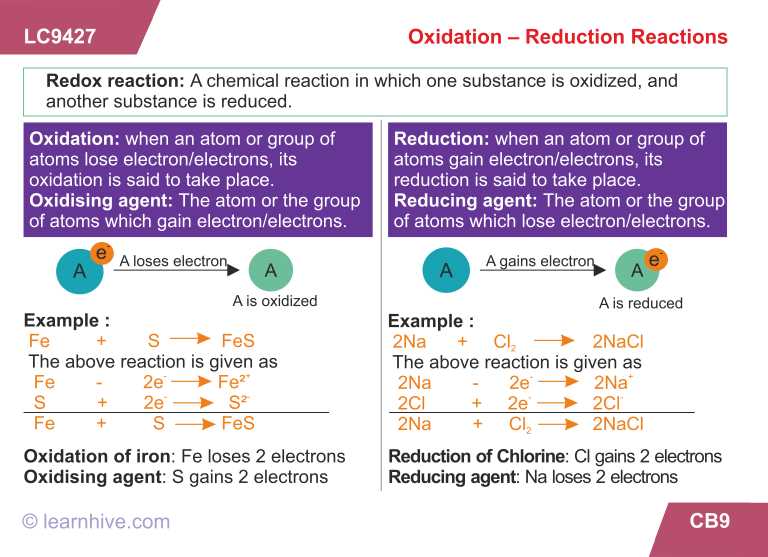
Ionic Bonds

Ionic bonds occur when one atom donates electrons to another atom, resulting in the formation of ions. In an ionic bond, the positively charged ion is called a cation, and the negatively charged ion is called an anion. These opposite charges attract each other, creating a strong bond. Ionic compounds typically have high melting and boiling points and conduct electricity when dissolved in water.
Covalent Bonds
Covalent bonds occur when atoms share electrons. These bonds are typically formed between nonmetal atoms. In a covalent bond, the electrons are shared unequally, creating a polar or nonpolar bond. Polar covalent bonds have a partial positive and partial negative charge, while nonpolar covalent bonds have an equal sharing of electrons. Covalent compounds have relatively low melting and boiling points and do not conduct electricity.
Metallic Bonds
Metallic bonds occur between metal atoms. In a metallic bond, the valence electrons are delocalized and move freely throughout a network of metal atoms. This results in the formation of a sea of electrons that hold the metal atoms together. Metallic compounds have high melting and boiling points and are good conductors of heat and electricity.
In summary, chemical bonding is the process by which atoms join together to form molecules and compounds. This can occur through ionic bonds, where electrons are transferred, covalent bonds, where electrons are shared, or metallic bonds, where electrons are delocalized. Understanding chemical bonding is crucial in understanding the properties and behavior of different substances.
Types of Chemical Bonds
Chemical bonds are the forces that hold atoms together in a compound. There are three main types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds. Each type of bond involves a different sharing or transfer of electrons between atoms.
Ionic Bonds
Ionic bonds occur when one or more electrons are transferred from one atom to another. This results in the formation of positive and negative ions that are attracted to each other. The atom that loses electrons becomes a positively charged ion, while the atom that gains electrons becomes a negatively charged ion. These oppositely charged ions then attract each other and form a bond.
Example: Sodium (Na) loses an electron to chlorine (Cl) to form sodium chloride (NaCl), a common salt.
Covalent Bonds
Covalent bonds occur when atoms share electrons to achieve a more stable electron configuration. In a covalent bond, the shared electrons are usually located in the outermost energy level of the atoms involved. The atoms that share electrons form a molecule. Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the atoms.
Example: In water (H2O), oxygen shares electrons with two hydrogen atoms to form a covalent bond.
Metallic Bonds
Metallic bonds occur between positively charged metal ions and a sea of delocalized electrons. In a metallic bond, the valence electrons are not associated with a specific atom but are free to move throughout the entire structure. This delocalization of electrons allows metals to conduct electricity and have unique properties like malleability and ductility.
Example: In a copper wire, copper atoms form metallic bonds with each other, creating a structure that allows the movement of electrons and thus, electrical conductivity.
In summary, ionic bonds involve the transfer of electrons, covalent bonds involve the sharing of electrons, and metallic bonds involve a sea of delocalized electrons. The type of bond formed depends on the electronegativity and properties of the atoms involved.
Ionic Bonding
Ionic bonding is a type of chemical bonding that occurs between atoms when one atom gains or loses electrons to form positive and negative ions. This type of bonding typically occurs between a metal and a nonmetal, where the metal atom loses electrons to form a positive ion, called a cation, and the nonmetal atom gains those electrons to form a negative ion, called an anion.
This transfer of electrons creates a strong attraction between the oppositely charged ions, known as an ionic bond. The positive and negative ions are held together by this electrostatic force, resulting in the formation of an ionic compound. Ionic compounds have a regular repeating pattern of ions, forming a crystal lattice structure.
For example: Sodium (Na) has one valence electron and is a metal, while chlorine (Cl) has seven valence electrons and is a nonmetal. Sodium donates its valence electron to chlorine, forming a sodium ion (Na+) and a chloride ion (Cl-). The positively charged sodium ion is attracted to the negatively charged chloride ion, creating an ionic bond. The resulting compound is sodium chloride (NaCl), commonly known as table salt.
Ionic compounds generally have high melting and boiling points due to the strong electrostatic forces between the ions. They are typically soluble in water and conductive when dissolved or molten, as the ions are able to move and carry electric charge. In solid state, however, ionic compounds are usually not conductive because the ions are fixed in their positions.
Overall, ionic bonding plays a crucial role in the formation of many minerals, salts, and other compounds. It is a fundamental concept in chemistry and understanding its principles helps explain the various properties and behaviors of ionic compounds.
Covalent Bonding
Covalent bonding is a type of chemical bonding where two atoms share one or more pairs of electrons. This type of bonding occurs between nonmetal atoms. In a covalent bond, both atoms contribute one or more electrons to the shared electron pair, forming a shared electron cloud around the bonded atoms.
Covalent bonds can be single, double, or triple bonds, depending on the number of electron pairs shared between the atoms. In a single covalent bond, one pair of electrons is shared, while in a double covalent bond, two pairs of electrons are shared. Likewise, in a triple covalent bond, three pairs of electrons are shared.
The strength of the covalent bond depends on the electronegativity difference between the atoms involved. If the electronegativity difference is small, the bond is considered nonpolar covalent, meaning electrons are shared equally between the atoms. If the electronegativity difference is large, the bond is considered polar covalent, meaning electrons are shared unequally and one atom has a stronger pull on the electron pair.
Covalent bonds are important in many biological and chemical processes. They help molecules maintain their shape and contribute to the physical and chemical properties of substances. Examples of substances held together by covalent bonds include water, carbon dioxide, and proteins.
Metallic Bonding
Metallic bonding is a type of chemical bonding that occurs between metal atoms. It is characterized by the sharing of electrons between metal atoms, resulting in a sea of delocalized electrons surrounding a lattice of positive metal ions. This unique bonding allows metals to exhibit their characteristic properties, such as high electrical and thermal conductivity, malleability, and ductility.
In a metallic bond, the valence electrons of metal atoms are loosely held and can move freely throughout the lattice. This delocalization of electrons allows for the conduction of electricity and heat. When a potential difference is applied, the delocalized electrons flow freely, carrying electric current. Similarly, the movement of these electrons allows metals to conduct heat efficiently.
The malleability and ductility of metals are also attributed to metallic bonding. The delocalized electrons provide a cushion between layers of metal ions, enabling the atoms to slide past each other without shattering the crystal lattice. This property allows metals to be easily shaped into different forms, such as sheets or wires, without breaking.
Overall, metallic bonding is essential for the unique properties and applications of metals. It enables metals to conduct electricity and heat, as well as be malleable and ductile. Understanding metallic bonding is crucial in fields such as materials science and engineering, where the properties of metals are utilized in various applications.
Hybridization in Chemical Bonds
Hybridization is a concept in chemistry that helps explain the molecular structure and bonding of different compounds. It is the combination of atomic orbitals in an atom to form new orbitals that are used for bonding. Hybrid orbitals have characteristics of both atomic orbitals and can overlap with other orbitals to form strong chemical bonds.
Hybridization plays a crucial role in determining the geometry and properties of molecules. The type of hybridization in a molecule can greatly influence its shape, polarity, and reactivity. It helps explain the observed bond angles and the formation of certain types of bonds, such as sigma and pi bonds.
There are different types of hybridization, including sp, sp2, and sp3. Each type is characterized by the combination of s and p orbitals to form hybrid orbitals. For example, sp hybridization involves the combination of one s orbital and one p orbital, resulting in two sp hybrid orbitals. These orbitals are oriented linearly, leading to a linear molecular geometry in the molecule.
In summary, hybridization is a fundamental concept in chemistry that helps explain the structure, bonding, and properties of molecules. It allows us to understand the geometries and characteristics of different compounds. By studying hybridization, researchers can better predict the behavior of molecules and design new compounds with desired properties.
Q&A:
What is hybridization in chemical bonds?
Hybridization in chemical bonds is a concept in which the atomic orbitals of different types hybridize to form new hybrid orbitals that influence the bonding and geometry of molecules.
What are the types of hybrid orbitals?
The types of hybrid orbitals include sp, sp2, and sp3, which correspond to one, two, and three p orbitals hybridizing with one s orbital, respectively.
Why does hybridization occur?
Hybridization occurs to minimize the energy of the molecule by allowing the electron density to be spread out over a larger space, resulting in more stable bonds and a well-defined geometry.
How does hybridization affect the shape of molecules?
Hybridization affects the shape of molecules by determining the arrangement of atoms and lone pairs around the central atom, which in turn influences the bond angles and molecular geometry.
What is the significance of hybridization in organic chemistry?
Hybridization is significant in organic chemistry because it explains the bonding and reactivity of organic compounds, helping to predict the types of bonds and angles formed in different molecules.
What is hybridization in chemical bonds?
Hybridization in chemical bonds is the mixing of atomic orbitals to form new hybrid orbitals. This process allows for the formation of stronger, more stable bonds.
What are the types of hybridization in chemical bonds?
There are several types of hybridization in chemical bonds, including sp, sp2, sp3, sp3d, and sp3d2. These types of hybridization are determined by the number of atomic orbitals that are mixed together.