
Understanding ionization energy is essential for any student studying chemistry. Ionization energy is defined as the amount of energy required to remove an electron from a gaseous atom or ion. It plays a crucial role in various chemical reactions and is an essential concept in understanding the periodic table and the behavior of elements.
Ionization energy can be determined experimentally by measuring the energy required to remove an electron from an atom or ion. However, it can also be calculated using the equation: Ionization Energy = – (E final – E initial), where E final is the energy of the ion or atom after the electron has been removed, and E initial is the energy of the ion or atom before the electron was removed.
One way for students to practice calculating ionization energy is by using a worksheet. A worksheet provides a structured way for students to apply their knowledge and practice solving problems related to ionization energy. An answer key for the worksheet helps students verify their solutions and identify any mistakes they may have made.
The answer key to an ionization energy worksheet typically includes the correct values for ionization energy for each element or ion, as well as step-by-step solutions for each problem. It helps students understand the process of calculating ionization energy and provides a useful tool for self-assessment and review.
What is Ionization Energy?
The ionization energy is the minimum amount of energy required to remove an electron from a neutral atom or ion in the gas phase. It is also known as ionization potential. This energy is typically measured in electron volts (eV) or kilojoules per mole (kJ/mol).
The ionization energy is an important property of an atom as it determines its ability to gain or lose electrons. It signifies how tightly an electron is held by the nucleus of an atom. If the ionization energy is high, it means that the electron is strongly held and it will require more energy to remove it. On the other hand, if the ionization energy is low, it means that the electron is weakly held and it will be easier to remove.
The ionization energy generally increases across a period from left to right in the periodic table as the atomic radius decreases and the nuclear charge increases. This results in a stronger attraction between the electrons and the nucleus, making it harder to remove an electron. However, there are a few exceptions to this trend, such as the irregularities in ionization energies of transition metals.
Ionization energy is also affected by factors such as the number of electrons in an atom, the shielding effect of inner electrons, and electron-electron repulsion. These factors can influence the ease with which an electron can be removed from an atom.
Understanding ionization energy is important in various fields of science, including chemistry, physics, and materials science. It helps in predicting the reactivity, stability, and behavior of atoms and molecules, as well as in the design and development of new materials and technologies.
Importance of Ionization Energy
Ionization energy is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of atoms and molecules. It is defined as the amount of energy required to remove an electron from an atom or ion in the gaseous state. Ionization energy is an essential property for predicting chemical reactivity and understanding the stability of atoms.
One of the primary applications of ionization energy is in the construction of the periodic table. The periodic table is organized based on increasing ionization energy, which reflects the trend in electron configuration and atomic properties. By arranging elements in order of their ionization energies, the periodic table provides a systematic way of understanding the periodic trends in properties such as atomic radius, electronegativity, and reactivity.
The concept of ionization energy is also crucial in the study of chemical bonding. In chemical reactions, atoms gain or lose electrons to form ions, and the ability to do so is determined by the ionization energy. High ionization energy indicates that an atom is less likely to lose an electron and form a positive ion, while low ionization energy suggests that an atom is more likely to gain an electron and form a negative ion.
Furthermore, ionization energy is closely related to the stability of atoms and the formation of chemical compounds. Elements with high ionization energies tend to have more stable electron configurations and are less likely to form compounds. On the other hand, elements with low ionization energies are highly reactive and readily form compounds with other elements.
In summary, ionization energy is a crucial concept in chemistry that helps explain the periodic trends in the properties of elements, determines chemical reactivity and bonding, and contributes to our understanding of the stability and behavior of atoms and molecules.
Factors Affecting Ionization Energy
The ionization energy is the energy required to remove an electron from an atom. This energy depends on several factors:
- Nuclear charge: The ionization energy generally increases as the nuclear charge or the number of protons in the nucleus increases. This is because the greater attraction between the positively charged nucleus and the negatively charged electron makes it more difficult to remove the electron.
- Atomic radius: The ionization energy decreases as the atomic radius increases. This is because the electron is held less tightly by the larger, more spread out electron cloud, making it easier to remove.
- Shielding effect: The ionization energy decreases as the number of electron shells or energy levels increases. This is because the outer electrons are shielded or partially blocked from the full attractive force of the nucleus by the inner electrons, making them easier to remove.
- Electron configuration: The ionization energy may vary depending on the specific electron configuration of an atom. For example, it generally requires more energy to remove an electron from a half-filled or completely filled orbital due to the increased stability of these configurations.
- Electron-electron repulsion: The ionization energy may also be influenced by electron-electron repulsion. For example, if there are two electrons in the same orbital, they will repel each other, making it slightly easier to remove one of the electrons.
Understanding these factors allows us to predict trends in ionization energy across the periodic table and analyze the stability and reactivity of different elements and compounds.
Ionization Energy Trend in the Periodic Table
The ionization energy trend in the periodic table refers to the pattern of how the ionization energy of an element changes as you move across or down the periodic table. Ionization energy, also known as ionization potential, is the amount of energy required to remove an electron from an atom or ion in its gaseous state. It is an important concept in understanding the chemical reactivity and behavior of elements.
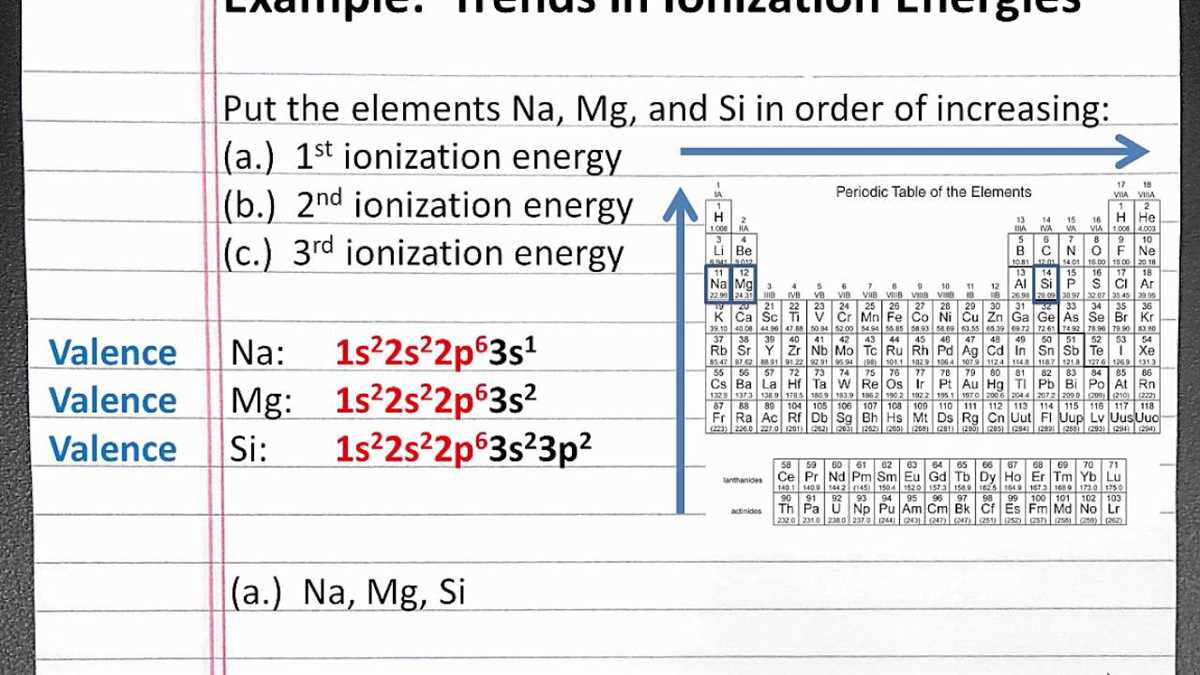
Across a period: In general, ionization energy tends to increase as you move from left to right across a period. This is because as you move across a period, the number of protons in the nucleus increases, resulting in a stronger attractive force between the nucleus and the electrons. As a result, it becomes more difficult to remove an electron, and therefore, the ionization energy increases.
Down a group: Ionization energy generally decreases as you move down a group in the periodic table. This is because the number of electron shells increases, shielding the outermost electrons from the attractive force of the nucleus. As a result, the outermost electrons are held less tightly and require less energy to remove, leading to a lower ionization energy.
- As you move from left to right across a period, the ionization energy increases.
- As you move down a group, the ionization energy decreases.
Understanding the ionization energy trend in the periodic table allows chemists to predict and explain the behavior and reactivity of elements. It also provides valuable information for various applications, such as designing materials with specific electronic properties and understanding the behavior of ions in chemical reactions. Overall, the ionization energy trend is a fundamental concept in chemistry that helps us understand the periodic behavior of elements.
Worksheet on Ionization Energy
Ionization energy is a key concept in the study of atoms and their behavior. It is defined as the amount of energy required to remove an electron from an atom or ion in a gaseous state. This energy is typically measured in units of kilojoules per mole (kJ/mol).
The worksheet on ionization energy provides students with a set of problems and questions related to this topic. It aims to help them understand the factors that influence ionization energy and practice calculating it for different elements.
Example question:
1. What is the ionization energy of an atom with an atomic number of 8?
- A. 520 kJ/mol
- B. 450 kJ/mol
- C. 1314 kJ/mol
- D. 496 kJ/mol
Answer key:
- D. 496 kJ/mol
This example question tests the student’s understanding of ionization energy and their knowledge of the periodic table. By completing the worksheet, students can strengthen their grasp of ionization energy and its role in the behavior of atoms.
Additional questions on the worksheet might include calculations of ionization energy for specific elements, comparisons of ionization energy across different elements, and explanations of the relationship between ionization energy and atomic structure.
Overall, the worksheet on ionization energy serves as a useful tool for students to practice and reinforce their understanding of this important concept in chemistry.
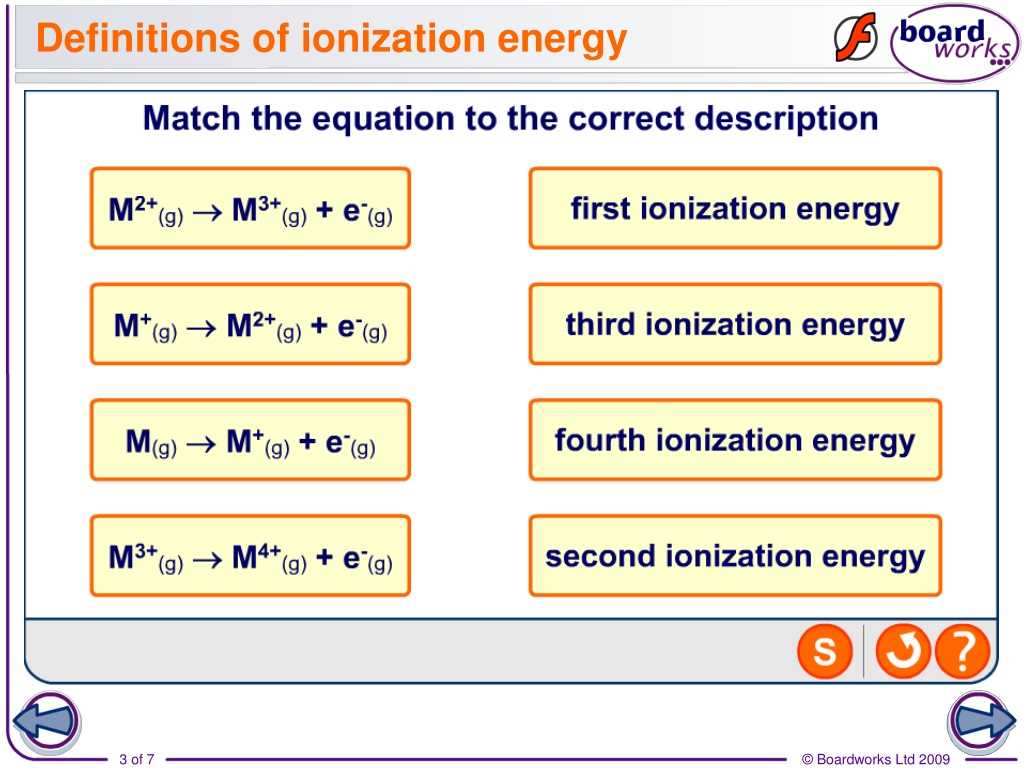
Answer Key for Ionization Energy Worksheet
In this worksheet, you were presented with various elements and their respective ionization energies. Your task was to determine the correct order of ionization energies based on the given values.
Here is the answer key to the worksheet:
Answer Key:
- Helium (He) – 24.6 eV
- Oxygen (O) – 13.6 eV
- Carbon (C) – 11.3 eV
- Aluminum (Al) – 5.9 eV
- Potassium (K) – 4.3 eV
- Sodium (Na) – 5.1 eV
- Fluorine (F) – 17.4 eV
- Neon (Ne) – 21.6 eV
- Lithium (Li) – 5.4 eV
- Magnesium (Mg) – 7.6 eV
It is important to understand that ionization energy is the amount of energy required to remove an electron from an atom or ion. Generally, ionization energy increases as you move from left to right across a period in the periodic table, and decreases as you move down a group.
This answer key should help you verify your answers and provide a better understanding of the concept of ionization energy. Keep practicing with similar worksheets to further enhance your knowledge in this area.