
Copper Silver Nitrate Reaction Lab is a commonly conducted experiment to understand the reaction between copper and silver nitrate. This lab helps students gain practical knowledge about chemical reactions, compounds, and the concept of oxidation and reduction. By observing the changes that occur during the experiment, students can deduce the answers to various questions related to the reaction.
During the lab, copper wire is placed in a beaker containing silver nitrate solution. As the reaction takes place, the copper wire begins to dissolve, and a reddish-brown precipitate is formed. This precipitate is a copper-silver compound, which is the result of the reaction. By carefully measuring the reactants and products, students can calculate the amount of copper wire that dissolved and the mass of the precipitate formed.
One of the questions that students need to answer in the lab is the balanced chemical equation for the reaction. By analyzing the reactants and products, students can deduce that the reaction is a displacement reaction, where copper displaces silver from silver nitrate. The balanced equation for this reaction is: 2AgNO3 + Cu → Cu(NO3)2 + 2Ag.
Another important question in the lab is the type of reaction that occurs. Based on the changes observed, students can conclude that the reaction is an oxidation-reduction reaction. Copper loses electrons and is oxidized, while silver ion gains electrons and is reduced. This reaction is also known as a redox reaction.
Background
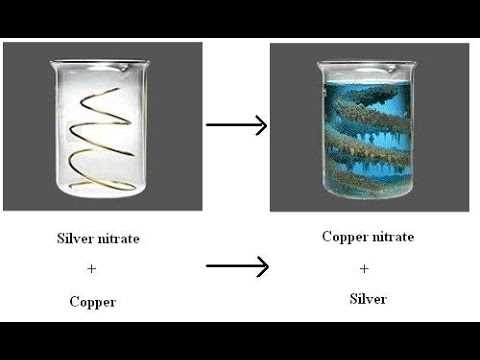
The copper-silver nitrate reaction is a popular chemistry experiment conducted in laboratories to illustrate various chemical concepts and principles. It involves the reaction between copper metal and silver nitrate solution, resulting in the formation of silver metal and copper(II) nitrate solution.
Copper and silver are both transition metals, belonging to Group 11 of the periodic table. They have similar electron configurations and exhibit similar chemical properties. One of the key concepts observed in this reaction is the displacement reaction, where an element of higher activity displaces an element of lower activity in a compound. In this case, silver, being higher on the activity series, displaces copper in the copper(II) nitrate compound, forming silver metal and copper(II) nitrate solution.
The reaction between copper and silver nitrate is also a redox reaction, involving both oxidation and reduction processes. Copper atoms are oxidized from a neutral state to a +2 oxidation state, while silver ions are reduced from a +1 oxidation state to neutral silver atoms. This electron transfer process is a fundamental principle in redox chemistry and is often encountered in various chemical reactions.
Students perform this reaction in the laboratory to observe the formation of a solid precipitate (silver metal), changes in color and appearance, and to practice various laboratory techniques such as measuring quantities, handling chemicals, and recording observations. It also provides an opportunity to analyze the reaction stoichiometry, calculate theoretical yields, and compare them with experimental results. In addition, this experiment allows students to apply their knowledge of chemical equations, balanced equations, and the concept of limiting reactants.
Experimental Procedure
The experiment is conducted by mixing copper wire with silver nitrate solution to observe the reaction between them. The following is the step-by-step procedure for conducting the experiment:
Materials:
- Copper wire
- Silver nitrate solution
- Beaker
- Stirring rod
- Dropper
- Filter paper
- Balance
- Bunsen burner
- Gauze
- Tongs
- Safety goggles
Procedure:
- Put on safety goggles and protective gloves to ensure personal safety.
- Weigh an appropriate amount of copper wire using a balance and record the mass.
- Using a dropper, carefully add silver nitrate solution to a beaker.
- Place the beaker on a gauze over a Bunsen burner and heat it until the solution is warm.
- Add the weighed copper wire to the warm silver nitrate solution and stir gently with a stirring rod.
- Observe the reaction between copper wire and silver nitrate solution. Note any color changes or formation of precipitate.
- After the reaction is complete, use tongs to remove the copper wire from the beaker and place it on filter paper to dry.
- Collect the formed precipitate on filter paper and allow it to dry as well.
- Weigh the dried copper wire and precipitate separately and record the mass.
- Analyze the data to determine the mass change and ratio of copper to silver in the reaction.
By following this experimental procedure, the reaction between copper wire and silver nitrate solution can be observed and analyzed to understand the chemical reaction that occurs between these substances.
Observations
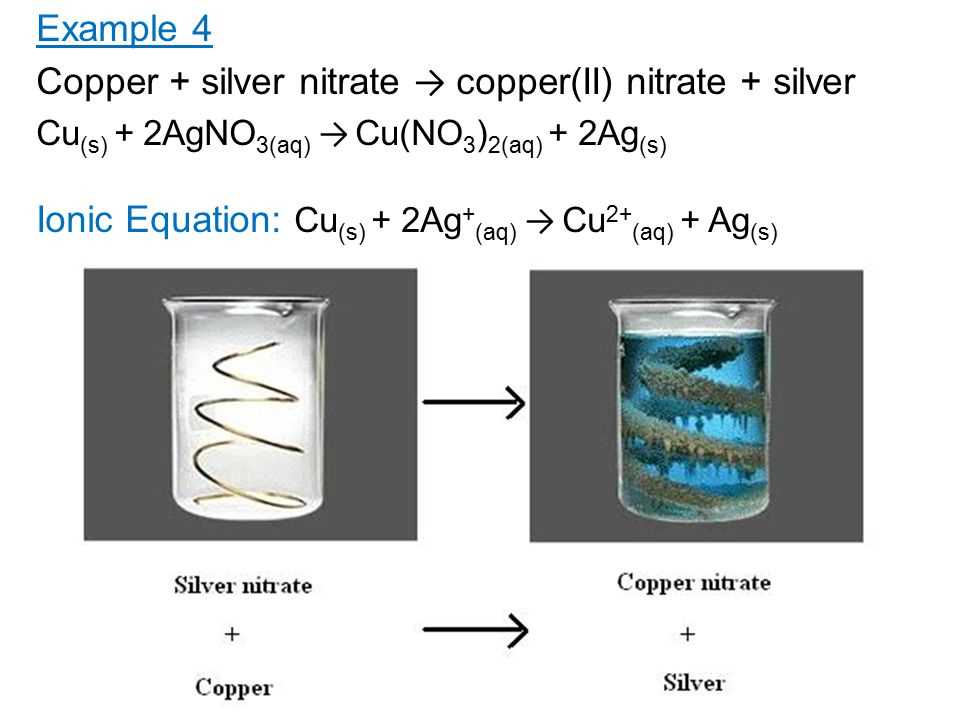
The copper silver nitrate reaction is a classic chemical reaction that involves the displacement of silver ions in silver nitrate solution by copper metal. This reaction is often used in laboratory experiments to demonstrate the reactivity of different metals and the concept of displacement reactions. During the reaction, several observations can be made.
Color change: Initially, the silver nitrate solution is colorless, while the copper metal is reddish-brown. As the reaction proceeds, the solution turns blue due to the formation of copper(II) nitrate, while the copper metal loses its reddish-brown color.
Precipitate formation: As the silver ions are displaced by copper, silver metal is formed as a precipitate. This can be observed as a grayish-black solid that settles at the bottom of the reaction vessel.
Gas evolution: Another observable phenomenon during the reaction is the evolution of gas. As the copper displaces the silver ions, nitrous oxide gas (N2O) is released. This gas can be identified by its characteristic sweet, ethereal smell.
Metallic appearance: Since the copper metal is being oxidized and dissolved into the solution, the remaining copper may appear dull or oxidized. This is due to the formation of copper(II) ions in the solution.
Temperature change: The reaction between copper and silver nitrate is exothermic, which means it releases heat. As a result, the reaction mixture may become warmer during the course of the reaction.
Overall, the copper silver nitrate reaction exhibits several observable changes, including color change, precipitate formation, gas evolution, metallic appearance, and temperature change. These observations help to understand the fundamental principles of chemical reactions and the reactivity of different elements.
Results and Discussion

The copper silver nitrate reaction lab experiment yielded interesting results. As expected, a redox reaction occurred between copper metal and silver nitrate solution. The copper metal displaced the silver ions in the solution, resulting in a copper nitrate solution and solid silver.
During the reaction, the color of the solution changed from clear to blue, indicating the formation of copper nitrate. The solid silver formed as a grayish-black precipitate at the bottom of the beaker. It had a metallic appearance and appeared to be pure silver.
The reaction proceeded rapidly, with the formation of copper nitrate and silver completed within a few minutes. It was observed that as the reaction progressed, the blue color of the solution intensified, indicating an increase in the concentration of copper ions.
Overall, the results of the copper silver nitrate reaction lab confirmed the expected outcome. The displacement reaction between copper metal and silver nitrate solution successfully demonstrated the redox reaction and the ability of copper to displace silver in a solution. The formation of copper nitrate and solid silver served as evidence of the reaction taking place.
These findings align with the principles of redox reactions and the reactivity series of metals. Copper, being higher in the reactivity series than silver, is capable of displacing silver ions. This experiment further reinforces the concept of displacement reactions and the behavior of metals in solution.