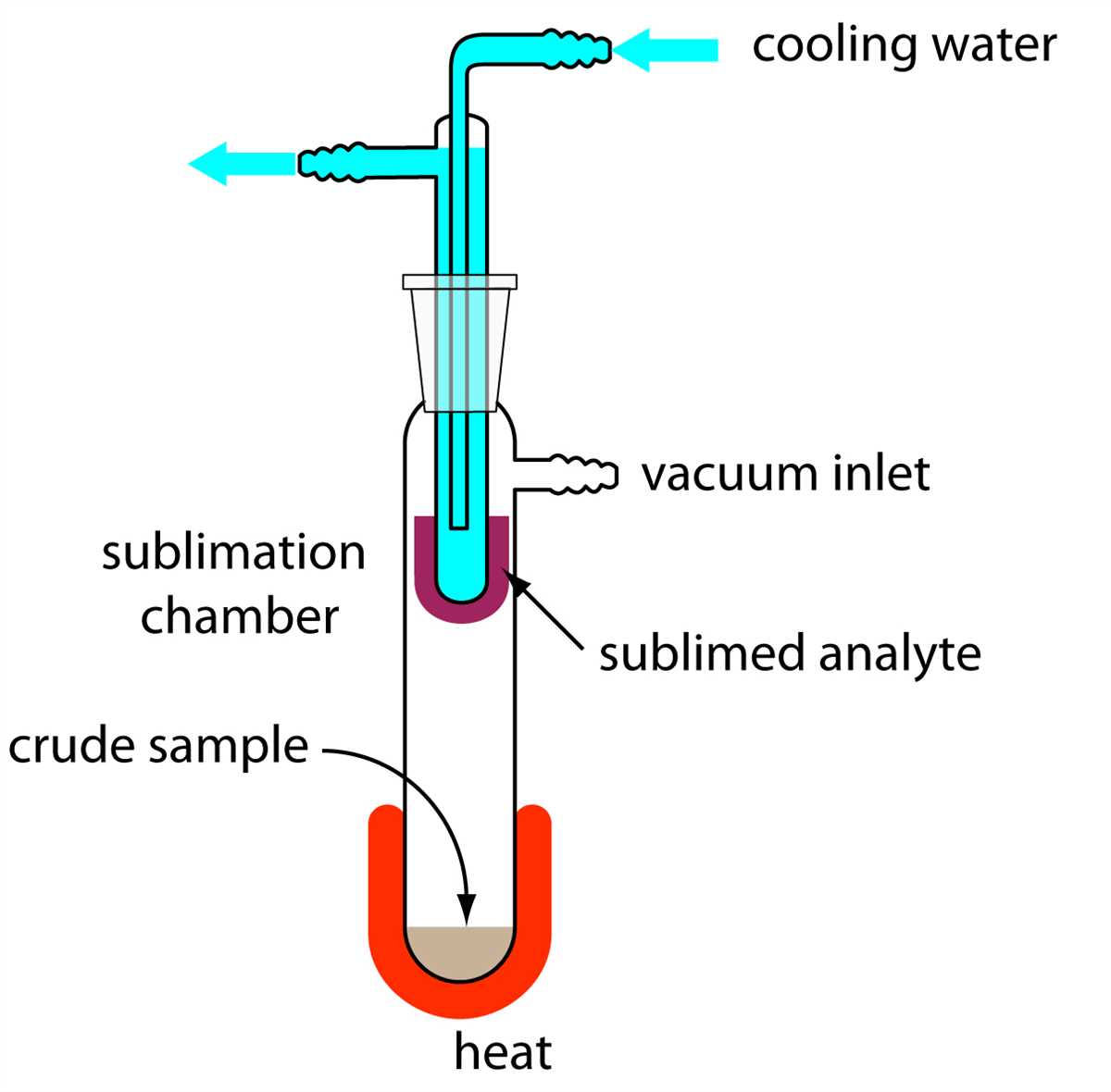
In the field of chemistry, mixtures are substances made up of two or more different components that are physically combined. Separating these components can be a challenging task, but it is a crucial step in understanding the properties of each individual substance. In this lab, we explored various techniques for separating mixtures and documented our findings.
One common method of separation is filtration. Filtration involves passing a mixture through a porous material, such as filter paper, to separate the solid components from the liquid. This technique is particularly useful when dealing with mixtures where one component is a solid and the other is a liquid. During the lab, we conducted filtration experiments and observed how the filter paper trapped the solid particles while allowing the liquid to pass through.
Another technique we explored was distillation. Distillation is a process that separates components of a mixture based on their boiling points. By heating the mixture and collecting the vapor that is produced, we were able to separate liquids with different boiling points. This method is commonly used for purifying and separating components from complex mixtures, such as crude oil.
What is a Separation of Mixtures Lab?
A separation of mixtures lab is a scientific laboratory experiment that involves the separation of different substances that are mixed together. It is often conducted to study and understand the properties and behavior of different materials in a mixture and to determine the most effective method of separating them. This type of lab is commonly performed in chemistry and biology classes, as well as in research and industrial settings, to isolate and analyze specific components of a mixture for further examination.
During a separation of mixtures lab, various techniques and methods are used to separate the different substances based on their physical or chemical properties. Some common separation techniques include filtration, distillation, chromatography, and evaporation. These techniques exploit differences in the boiling point, solubility, size, or magnetism of the substances in the mixture to isolate them.
Filtration is a method used to separate solid particles from a liquid or gas by passing the mixture through a filter, which retains the solid particles and allows the liquid or gas to pass through. It is often used to separate insoluble solids from liquids or gases.
Distillation is a technique that involves heating a mixture to vaporize a volatile component, then condensing the vapor and collecting it as a liquid. It is commonly used to separate substances with different boiling points, such as a mixture of liquids.
Chromatography is a method that involves the separation of substances based on their differential migration through a medium, such as a solid or liquid phase. It is often used to separate mixtures of different compounds, such as dyes, pigments, or chemicals.
Evaporation is a process in which a liquid is converted into a vapor state by heating or exposing it to low pressure. It is often used to separate dissolved solids or solutes from a liquid by evaporating the liquid and leaving the solid behind.
In conclusion, a separation of mixtures lab is an essential tool in scientific research and education. It allows scientists and students to study the properties of different substances in a mixture and develop effective methods for separating them. By understanding the principles and techniques involved in separation, scientists can better analyze and purify mixtures for various applications in fields such as healthcare, environmental science, and materials science.
Importance of Separating Mixtures in a Lab
Mixtures are combinations of two or more substances that can be separated through various processes. In a laboratory setting, separating mixtures is an essential step in analytical chemistry. It enables scientists to isolate and identify the individual components of a mixture, which is crucial for conducting accurate experiments and obtaining reliable results.
One of the primary reasons for separating mixtures in a lab is to study the properties of each substance independently. By isolating the different components, scientists can determine their physical and chemical characteristics, such as boiling points, melting points, solubility, and reactions with other substances. This information is valuable in understanding the behavior and nature of each component, which can then be applied to different fields of study, including pharmaceuticals, environmental sciences, and materials science.
1. Ensuring Accuracy and Precision
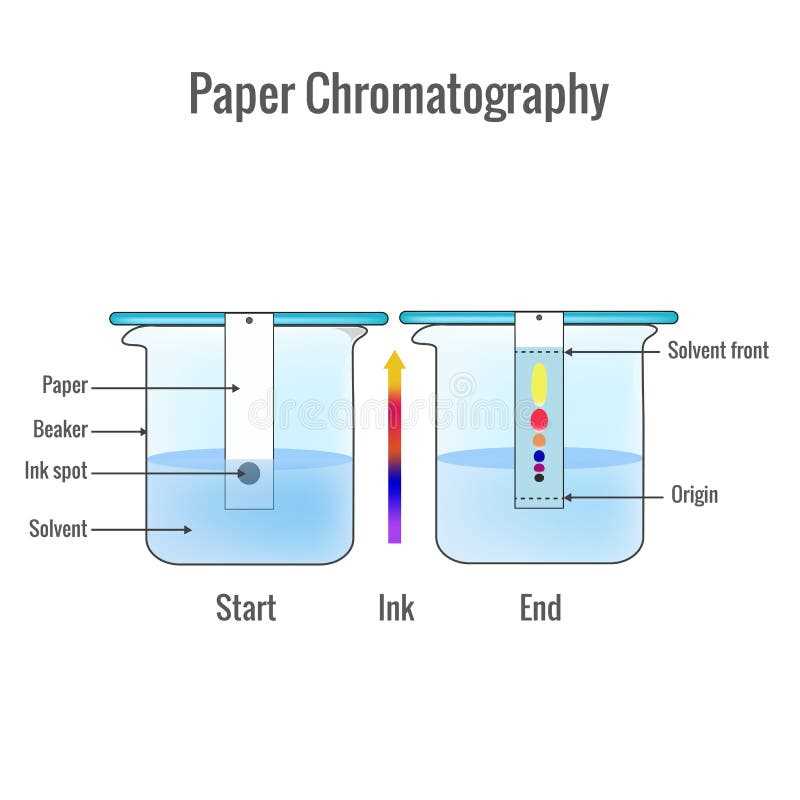
In a lab, separating mixtures is crucial for ensuring accuracy and precision in scientific experiments. When working with mixtures, they can contain impurities or contaminants that affect the results. By separating the mixture, scientists can eliminate these interferences and obtain a pure sample of each component. This allows for precise measurements, calculations, and analysis, leading to reliable and reproducible data.
2. Identifying Unknown Substances
Separating mixtures also plays a vital role in identifying unknown substances. In analytical chemistry, it is common to encounter samples with multiple components whose identities are not known. By employing separation techniques, such as chromatography or distillation, scientists can separate the mixture into its individual components. These components can then be analyzed further using various spectroscopic or analytical methods to determine their identities. This process is crucial in forensic analysis, drug testing, and quality control in industries.
3. Purification and Extraction
Separating mixtures is often done to purify substances or extract valuable compounds from a mixture. There are numerous techniques available, such as filtration, evaporation, and crystallization, that allow for the isolation of pure substances. Purification and extraction are important in industries like food processing, pharmaceuticals, and chemical manufacturing. They ensure the removal of undesired impurities and enhance the quality and potency of the final product.
In conclusion, separating mixtures in a lab is of utmost importance for accurate analysis, identification, and purification of substances. It enables scientists to gain a deeper understanding of the individual components within a mixture and apply this knowledge to various fields of research and industry. It is a critical step in ensuring the reliability and validity of scientific experiments and discoveries.
Methods Used in Separation of Mixtures
Mixtures are combinations of different substances that are physically combined. They can be separated into their constituent parts through various methods. One commonly used method is filtration. Filtration is the process of separating solid particles from a liquid or gas by passing it through a porous material such as filter paper. This method is particularly useful for separating insoluble solids from liquids, such as removing dirt or sediment from water.
Another method used in separation of mixtures is distillation. Distillation is a process that utilizes the differences in boiling points of the components in a mixture. The mixture is heated to its boiling point, and the vapor is collected and condensed back into a liquid. This method is frequently used for separating liquids from solutions or for obtaining pure liquids from mixtures.
One more common method is extraction. Extraction involves the separation of one or more components from a mixture by using a solvent that can dissolve the desired component. The mixture is mixed with the solvent, and the desired component is dissolved in the solvent. The mixture is then filtered, and the solvent is evaporated to obtain the desired component in its pure form.
Other methods used in separation of mixtures include crystallization, chromatography, and centrifugation. Crystallization involves the formation of solid crystals from a solution, which can then be separated from the remaining liquid. Chromatography is a technique used to separate the components of a mixture based on their different affinities for a stationary phase and a mobile phase. Centrifugation is the process of separating solid or liquid particles from a suspension by spinning it rapidly, causing the denser particles to settle at the bottom.
Common Apparatus and Equipment Used in Separation of Mixtures
The separation of mixtures is a common practice in various laboratory settings. In order to achieve efficient separation, several apparatus and equipment are used. These tools are specifically designed to aid in the separation process and allow for the isolation of individual components within a mixture.
Here are some common apparatus and equipment used in the separation of mixtures:
- Filtration apparatus: This apparatus consists of a funnel, filter paper, and a flask. It is used to separate solid particles from a liquid by passing the mixture through a porous medium, such as filter paper.
- Centrifuge: A centrifuge is a machine that spins samples at high speeds, creating a centrifugal force. It is used to separate components of a mixture based on their different densities. The heavier components settle at the bottom while the lighter components stay on top.
- Evaporating dish: An evaporating dish is a shallow, flat-bottomed glass dish used to evaporate the solvent from a solution. It is heated gently to allow the liquid to evaporate, leaving behind the solute.
- Distillation apparatus: Distillation is a technique used to separate two or more liquids with different boiling points. The apparatus consists of a distillation flask, a condenser, and a receiver. The mixture is heated, and the vapors are condensed and collected in the receiver.
- Decantation flask: A decantation flask is used to separate immiscible liquids or liquids and solids that have settled into layers. The liquids are poured off, leaving the solid or the less dense liquid behind.
These are just a few examples of the apparatus and equipment commonly used in the separation of mixtures. Each tool serves a specific purpose and allows scientists and laboratory technicians to separate and isolate the various components of a mixture efficiently and accurately.
Step-by-Step Procedure for a Separation of Mixtures Lab
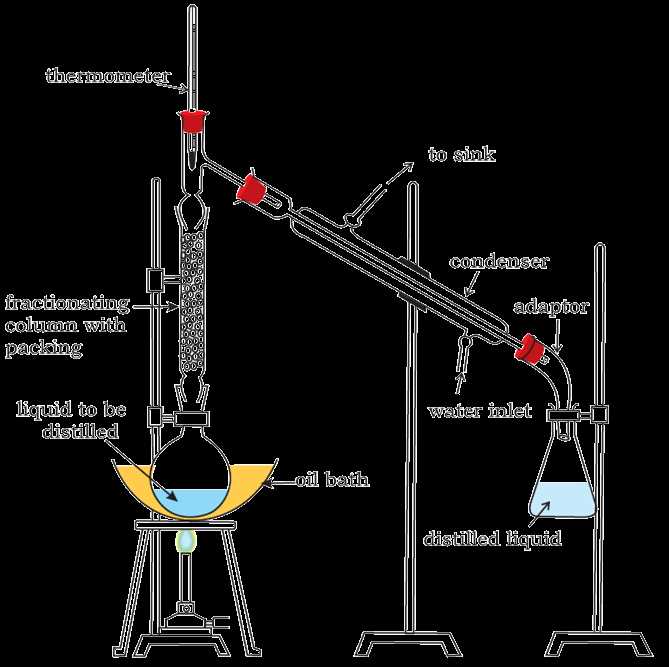
In a separation of mixtures lab, the objective is to separate a mixture into its individual components using various techniques. The following step-by-step procedure outlines the general process for conducting a separation of mixtures lab:
Materials:
- Mixture to be separated
- Distilled water
- Filter paper
- Beakers
- Funnel
- Magnets (if applicable)
- Heat source (if applicable)
- Test tubes
- Test tube rack
- Centrifuge (if applicable)
- Other necessary lab equipment
Procedure:
- Observe the mixture and identify the different components present.
- If the mixture contains solid and liquid components, start by using filtration to separate them. Set up a funnel and place a piece of filter paper inside it. Pour the mixture through the funnel and collect the filtrate (liquid) in a beaker. The solids will remain on the filter paper.
- If the mixture contains magnetic and non-magnetic components, use a magnet to separate them. Pass the magnet over the mixture, and the magnetic components will be attracted to it, allowing for easy separation.
- If the mixture contains components with different boiling points, use distillation to separate them. Heat the mixture in a distillation apparatus, and collect the condensate in a separate container. The component with the lower boiling point will vaporize and condense first, allowing for separation.
- If the mixture contains components that are soluble in different solvents, use extraction to separate them. Add a solvent to the mixture and shake it well. The different components will dissolve in different solvents, allowing for separation.
- If the mixture contains components of different densities, use centrifugation to separate them. Place the mixture in a test tube and spin it in a centrifuge. The components will separate based on their densities, with the heavier component settling at the bottom of the test tube.
- Once the mixture has been separated into its individual components, observe and analyze each component to confirm the success of the separation.
Conclusion:
By following this step-by-step procedure, a separation of mixtures lab can effectively separate a mixture into its individual components. The specific techniques used will depend on the nature of the mixture and its components. It is important to carefully observe and analyze the results to ensure a successful separation. Separating mixtures can be a valuable technique in various fields, including chemistry, biology, and environmental science.