
If you are studying organic chemistry, you know that sn1 and sn2 reactions are essential topics to master. These reactions involve the substitution of one atom or group with another atom or group in a molecule. Understanding the mechanisms and factors that influence these reactions is crucial for success in the field.
To improve your understanding and proficiency in sn1 and sn2 reactions, it is important to practice solving problems. By working on a variety of problems, you can identify patterns, develop strategies, and reinforce your knowledge of the reactions.
This article provides a collection of sn1 and sn2 practice problems with answers in PDF format. These problems cover a range of difficulty levels and scenarios, allowing you to test your understanding and problem-solving skills. Whether you are preparing for an exam or simply want to strengthen your knowledge, these practice problems will be a valuable resource.
Understanding Sn1 and Sn2 reactions
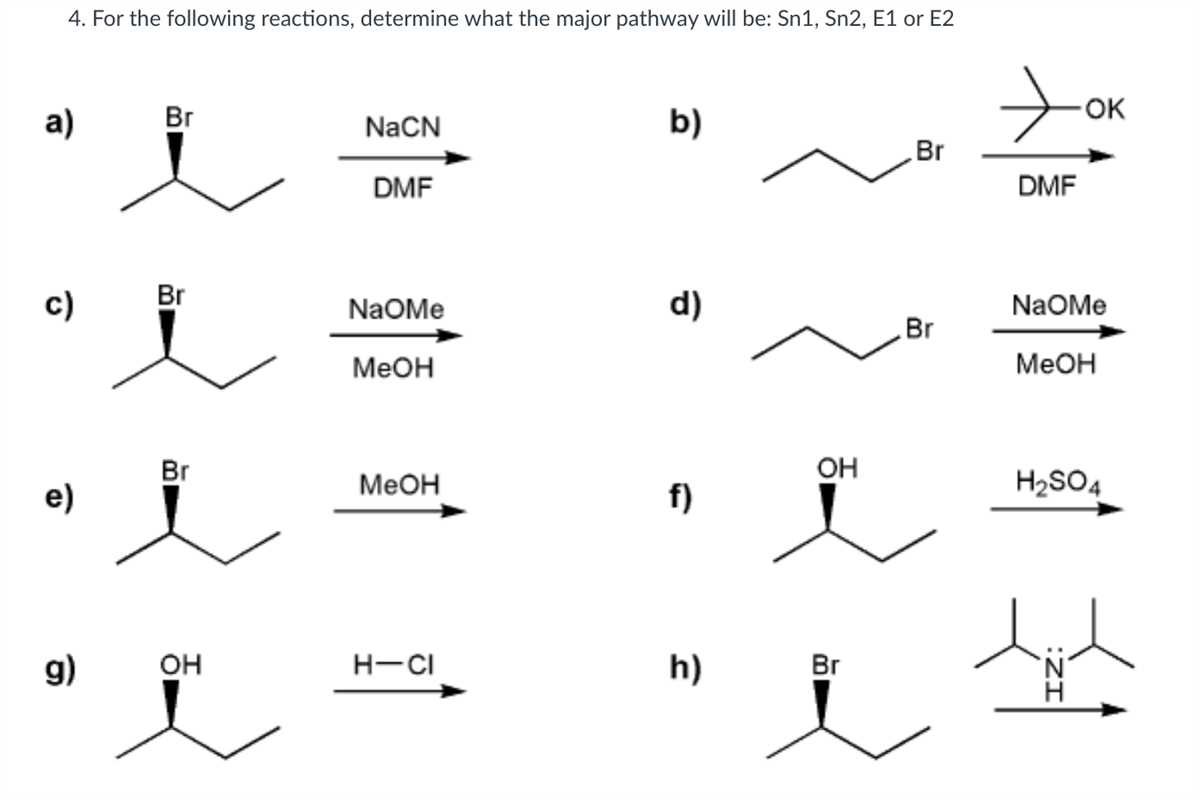
In organic chemistry, substitution reactions are a fundamental concept to understand. Two types of substitution reactions that frequently occur are Sn1 (substitution nucleophilic unimolecular) and Sn2 (substitution nucleophilic bimolecular) reactions. These reactions involve the replacement of one functional group in a molecule with another functional group.
The Sn1 reaction involves a two-step process. In the first step, the leaving group dissociates from the substrate, forming a carbocation intermediate. In the second step, the nucleophile attacks the carbocation, resulting in the formation of the substitution product. The rate of the Sn1 reaction depends only on the concentration of the substrate, as the nucleophile is not involved in the rate-determining step.
The Sn2 reaction, on the other hand, involves a one-step process. The nucleophile directly attacks the substrate, causing the leaving group to dissociate. The rate of the Sn2 reaction depends on both the concentration of the substrate and the nucleophile, as both are involved in the rate-determining step. The stereochemistry of the reaction is also important, as the nucleophile approaches the substrate from the backside, resulting in an inversion of configuration.
Understanding the differences between Sn1 and Sn2 reactions is crucial in predicting the outcome of a substitution reaction. Factors such as the nature of the substrate, the strength of the nucleophile, and the solvent used can all influence the reaction mechanism. By practicing Sn1 and Sn2 reaction problems and studying their outcomes, organic chemists can develop a deeper understanding of these reactions and apply them in various synthesis and reaction mechanisms.
Common substrates and nucleophiles encountered in Sn1 and Sn2 reactions
Sn1 and Sn2 reactions are two important types of nucleophilic substitution reactions in organic chemistry. In these reactions, a nucleophile replaces a leaving group on a substrate, resulting in the formation of a new compound. Understanding the common substrates and nucleophiles encountered in Sn1 and Sn2 reactions is crucial for predicting and understanding the outcome of these reactions.
Common substrates:
- Alkyl halides: Alkyl halides are commonly used as substrates in Sn1 and Sn2 reactions. They typically have a halogen (such as chlorine, bromine, or iodine) attached to an alkyl group.
- Alcohols: Alcohols can also serve as substrates in Sn1 and Sn2 reactions. They are often converted into alkyl halides before undergoing nucleophilic substitution.
- Epoxides: Epoxides, or oxiranes, are three-membered cyclic ethers. They can undergo Sn1 and Sn2 reactions to open the ring and form new compounds.
Common nucleophiles:
- Alkoxides: Alkoxides are nucleophiles derived from alcohols by replacing the hydrogen atom of the hydroxyl group with an alkyl group. They can participate in Sn2 reactions.
- Halides: Halides, such as chloride, bromide, and iodide ions, can act as nucleophiles in Sn2 reactions. They attack the substrate and replace the leaving group.
- Amines: Amines, compounds containing a nitrogen atom with one or more alkyl or aryl groups attached, can also act as nucleophiles in Sn2 reactions. They are often used in organic synthesis.
It is important to note that the choice of substrate and nucleophile can greatly influence the mechanism and rate of the nucleophilic substitution reaction. Factors like the nature of the leaving group, the steric hindrance, and the solvent used also play a role in determining the outcome of Sn1 and Sn2 reactions. By studying and understanding the common substrates and nucleophiles encountered in Sn1 and Sn2 reactions, chemists can make predictions and design reactions for specific organic synthesis purposes.
Distinguishing between Sn1 and Sn2 reactions

Sn1 and Sn2 reactions are both types of nucleophilic substitution reactions that occur in organic chemistry. However, they differ in terms of their reaction mechanisms and the conditions under which they occur.
Sn1 reactions: Sn1 reactions are characterized by a two-step mechanism. In the first step, the leaving group leaves the molecule, generating a carbocation intermediate. In the second step, the nucleophile attacks the carbocation, resulting in the substitution of the leaving group by the nucleophile. Sn1 reactions typically occur in polar protic solvents and involve tertiary or secondary substrate molecules. They are favored by heat and have a unimolecular rate equation.
Sn2 reactions: Sn2 reactions, on the other hand, follow a one-step mechanism. In this mechanism, the nucleophile directly attacks the substrate molecule at the same time as the leaving group is leaving. Sn2 reactions typically occur in polar aprotic solvents and involve primary or methyl substrate molecules. They are favored by cold temperatures and have a bimolecular rate equation.
There are several key factors that can help distinguish between Sn1 and Sn2 reactions:
- Substrate type: Sn1 reactions occur with tertiary or secondary substrates, while Sn2 reactions occur with primary or methyl substrates.
- Nucleophile strength: Sn1 reactions are not highly dependent on nucleophile strength, while Sn2 reactions require a strong nucleophile.
- Solvent type: Sn1 reactions occur in polar protic solvents, while Sn2 reactions occur in polar aprotic solvents.
- Temperature: Sn1 reactions are favored by heat, while Sn2 reactions are favored by cold temperatures.
- Reaction rate: Sn1 reactions have a unimolecular rate equation, while Sn2 reactions have a bimolecular rate equation.
By considering these factors, it is possible to differentiate between Sn1 and Sn2 reactions, allowing chemists to predict the reaction mechanism and conditions for a given reaction.
Problem-solving approach for Sn1 and Sn2 reactions
Sn1 and Sn2 reactions are important concepts in organic chemistry that involve the substitution of a nucleophile for a leaving group on a carbon atom. These reactions can be challenging to understand and predict, but by applying a problem-solving approach, you can improve your understanding and ability to solve Sn1 and Sn2 reaction problems.
The first step in solving Sn1 and Sn2 reaction problems is to identify the type of reaction you are dealing with. Sn1 reactions involve a two-step mechanism, where the leaving group dissociates first to form a carbocation, followed by the attack of the nucleophile. Sn2 reactions, on the other hand, involve a one-step mechanism, where the nucleophile directly attacks the carbon atom with the leaving group, causing a simultaneous bond formation and bond breaking.
Once you have identified the type of reaction, the next step is to analyze the reactants and products to determine the key factors that influence the reaction. These factors include the nature of the leaving group, the strength of the nucleophile, the steric hindrance around the carbon atom, and the solvent used. By considering these factors, you can make predictions about the outcome of the reaction and the relative rates of Sn1 and Sn2 pathways.
Another important step in problem-solving Sn1 and Sn2 reactions is to practice with a variety of examples and exercises. This will help you become familiar with the different scenarios that can occur and improve your ability to predict the outcome of the reaction. You can find Sn1 and Sn2 practice problems with answers in PDF format, which provide a valuable resource for honing your problem-solving skills.
In conclusion, by applying a problem-solving approach, identifying the type of reaction, analyzing the key factors, and practicing with examples, you can improve your understanding and ability to solve Sn1 and Sn2 reaction problems. With practice, you will become more confident in predicting the outcome of these reactions and successfully solving related problems.
Practice Problems: Sn1 Reactions
In organic chemistry, substitution reactions are an important topic to understand. One type of substitution reaction is called Sn1 reaction, which stands for nucleophilic substitution unimolecular. Sn1 reactions involve a two-step process where the leaving group first leaves the substrate, forming a carbocation intermediate, and then the nucleophile attacks the carbocation to form the final product.
It is essential to practice solving Sn1 reaction problems to gain a better understanding of the reaction mechanism and to improve problem-solving skills. Here are some practice problems to help you test your knowledge and proficiency in Sn1 reactions:
- Problem 1: Predict the major product of the following Sn1 reaction:
- Substrate: 2-bromo-2-methylbutane
- Nucleophile: Water (HO-)
- Problem 2: Determine the rate-determining step in the following Sn1 reaction:
- Substrate: 3-chloro-3-methylpentane
- Nucleophile: Methanol (CH3OH)
- Problem 3: Identify the intermediate formed in the following Sn1 reaction:
- Substrate: 1-chlorobutane
- Nucleophile: Iodide ion (I-)
By practicing these Sn1 reaction problems and understanding the concepts behind them, you will become more proficient in predicting products, identifying reaction intermediates, and determining the rate-determining steps in Sn1 reactions. This will ultimately enhance your overall understanding of organic chemistry and help you excel in your studies and future endeavors.
Practice problems: Sn2 reactions
In this section, we will discuss some practice problems related to Sn2 reactions. These problems will help you further understand the concept and enhance your problem-solving skills.
Here are some Sn2 reaction practice problems:
-
Problem 1: Predict the product for the following Sn2 reaction:
Starting material: CH3CH2Br
Reagent: NaOH
-
Problem 2: Identify the nucleophile and leaving group in the following Sn2 reaction:
Reaction: CH3CH2I + OH- ⟶ CH3CH2OH + I-
-
Problem 3: Determine the stereochemistry of the following Sn2 reaction:
Starting material: (R)-2-bromopentane
Reagent: NaOH
Product:
Don’t worry if you’re unsure about the answers. Practice these problems, refer to your study materials, and review the Sn2 reaction mechanism to reinforce your understanding. It’s a gradual learning process that improves with practice and repetition.
Remember, Sn2 reactions involve the substitution of nucleophiles with leaving groups, resulting in the inversion of stereochemistry. By practicing these problems, you’ll become more proficient in identifying reactants, products, and reaction mechanisms associated with Sn2 reactions.