
In chemistry, the concentration of a solution refers to the amount of solute present in a given volume of solvent. It is an important concept because it helps us understand the properties and behavior of solutions. In this article, we will discuss the key concepts and calculations related to concentrations of solutions.
One commonly used unit to express concentration is molarity (M). Molarity represents the number of moles of solute in one liter of solution. To calculate the molarity of a solution, we divide the number of moles of solute by the volume of the solution in liters.
Another unit of concentration is molality (m). Molality is defined as the number of moles of solute per kilogram of solvent. Unlike molarity, which takes into account the volume of the solution, molality only considers the mass of the solvent.
Concentrations of solutions can also be expressed in terms of percent by mass or percent by volume. Percent by mass is calculated by dividing the mass of the solute by the mass of the solution and multiplying by 100. Percent by volume, on the other hand, is calculated by dividing the volume of the solute by the volume of the solution and multiplying by 100.
Understanding concentrations of solutions is crucial in various applications, such as pharmaceuticals, environmental science, and industrial processes. It helps scientists and engineers determine the appropriate amount of solute needed for a desired effect and ensures the accuracy and efficacy of the solutions used.
Understanding concentrations in solutions
Concentrations are an important concept in chemistry and are used to describe the amount of solute dissolved in a solvent. The concentration of a solution can be expressed in various units, such as molarity, mass/volume percent, and mole fraction. Understanding concentrations in solutions is crucial for many applications, including pharmaceuticals, environmental science, and industrial processes.
One commonly used unit of concentration is molarity (M), which represents the number of moles of solute per liter of solution. It is calculated by dividing the moles of solute by the volume of the solution in liters. Molarity is widely used because it allows for easy conversion between mass and volume. For example, in a 1 M solution of sodium chloride, there is 58.44 grams of sodium chloride per liter of solution.
Another unit of concentration is mass/volume percent, which represents the mass of the solute divided by the volume of the solution, multiplied by 100. This unit is commonly used in the pharmaceutical industry to express the concentration of drugs in solutions. For example, a 5% glucose solution means that there are 5 grams of glucose dissolved in 100 milliliters of solution.
Understanding concentrations in solutions is essential for performing accurate experiments and calculations in chemistry. It allows scientists to determine the amount of solute needed to prepare a specific concentration of solution and helps in conducting reactions with the desired concentration. Additionally, understanding concentrations helps scientists analyze the behavior of solutions, such as the rate of reactions and the solubility of substances.
In conclusion, concentrations in solutions are a vital component of chemistry and have numerous applications in various fields. Whether it is measuring the concentration of pollutants in environmental samples or preparing a specific concentration of a drug, understanding concentrations is essential for accurate experimentation and analysis.
Calculating concentrations in solutions

Concentration is a fundamental concept in chemistry, as it allows us to quantify the amount of a solute dissolved in a given amount of solvent. It is typically expressed as the amount of solute per unit volume or mass of solvent. Calculating concentrations is crucial for various applications, such as determining the strength of a medication, measuring pollutant levels in water, or preparing solutions for experiments.
There are several ways to express concentration, depending on the properties of the solute and the solvent. One common unit is molarity (M), which represents the number of moles of solute per liter of solution. To calculate molarity, we need to know the mass of the solute and the volume of the solution. By using the equation Molarity (M) = moles of solute / volume of solution (L), we can determine the concentration in moles per liter.
Another commonly used unit for concentration is percent by mass or volume. Percent by mass represents the mass of the solute divided by the total mass of the solution, multiplied by 100%. Percent by volume, on the other hand, represents the volume of the solute divided by the total volume of the solution, multiplied by 100%. These units are often used in everyday applications, such as expressing the concentration of alcohol in a beverage or the concentration of oxygen in the air we breathe.
It is also important to consider dilution calculations, which involve changing the concentration of a solution by adding more solvent. In dilution calculations, we can use the equation initial concentration x initial volume = final concentration x final volume. This equation allows us to determine the amount of solute or solvent needed to obtain a desired concentration.
In conclusion, calculating concentrations in solutions is a vital skill in chemistry that allows us to measure and manipulate the amount of solute in a given solvent. Whether it’s measuring molarity, percent by mass or volume, or performing dilution calculations, understanding and applying these concepts is essential for many chemical processes and experiments.
Methods of expressing concentrations in solutions
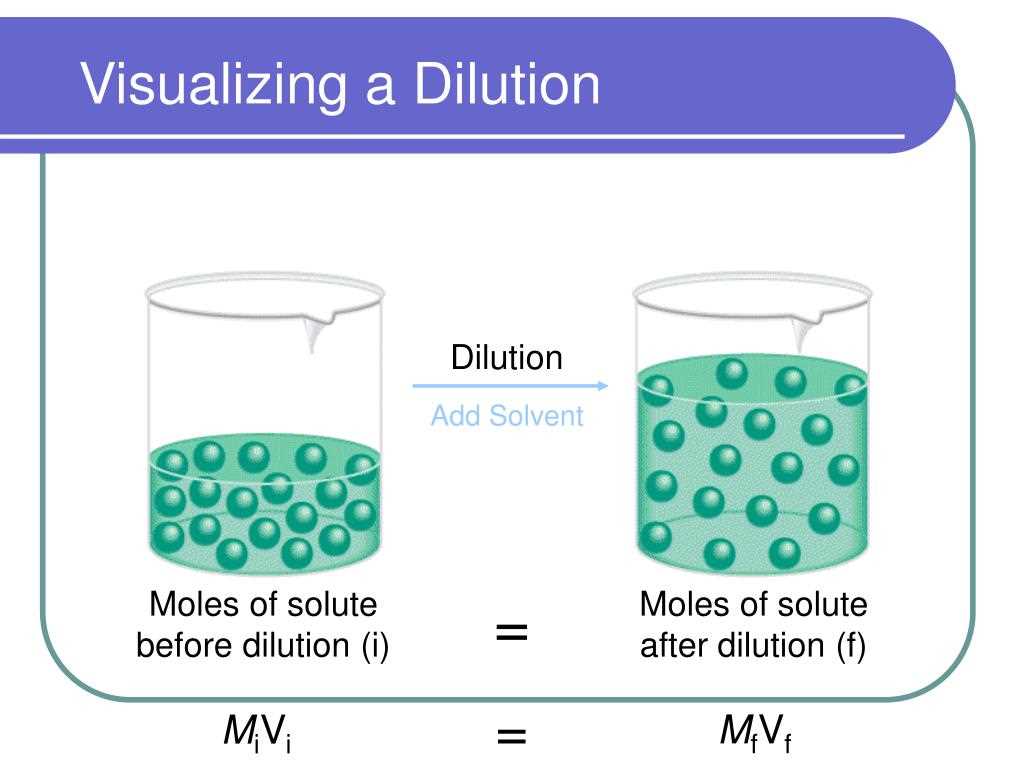
In chemistry, concentrations of solutions are often expressed in various units to provide specific and meaningful information about the amount of solute present in a given volume of solvent. These methods of expressing concentrations help chemists in accurately measuring, comparing, and communicating information about the strength or dilution of solutions.
Dilution refers to the process of reducing the concentration of a solute in a solution by adding more solvent. It is often expressed as the ratio of the volume of the solute to the total volume of the solution. For example, a 1:10 dilution means that 1 part of the solute is mixed with 10 parts of the solvent, resulting in a 1/11 concentration of the solute in the final solution.
Molarity is one of the most commonly used methods of expressing concentration. It is defined as the number of moles of solute present in one liter of solution. Molarity is denoted by the symbol “M”. For example, a solution with a molarity of 0.5 M means that there are 0.5 moles of solute dissolved in every liter of the solution. Molarity is an important unit in chemical reactions as it helps in calculating the amount of reactants or products needed or produced.
- Percentage by mass is another method of expressing concentration and is often used when the solute is a solid. It represents the mass of the solute divided by the mass of the entire solution, multiplied by 100. For example, a 5% solution of sodium chloride means that 5 grams of sodium chloride is dissolved in every 100 grams of the solution.
- Parts per million (ppm) is a unit used to express extremely small concentrations, especially for pollutants or contaminants. It represents the ratio of the mass of solute to the mass of the solution, multiplied by 1 million. For example, a water sample with a concentration of 10 ppm of lead means that there are 10 milligrams of lead dissolved in every kilogram of water.
These are just a few examples of the methods used to express concentrations in solutions. Each method provides specific information that is useful in different contexts and applications within chemistry.
The Importance of Knowing Concentrations in Solutions

Concentration is a crucial factor to consider when working with solutions in various fields, including chemistry, biology, and medicine. It refers to the amount of solute present in a given volume of solvent. Understanding concentrations is essential for accurate measurements, proper dosing, and effective analysis.
In analytical chemistry, knowing the concentration of a solution is essential for quantifying the amount of a particular substance. This is especially important when conducting experiments, quality control tests, and research studies. By accurately measuring the concentration, scientists can determine the exact amount of a substance present, allowing for precise calculations and comparisons between different samples.
In the field of medicine, concentration plays a critical role in drug administration. Doctors and pharmacists need to know the concentration of a drug solution to determine the appropriate dosage for patients. Incorrect concentrations can lead to ineffective treatment or even harmful side effects. Additionally, knowing the concentration of certain substances in the body, such as blood glucose levels, is crucial for diagnosing and managing medical conditions.
Industrial processes also heavily rely on knowing concentrations in solutions. From manufacturing to wastewater treatment, precise measurements of concentrations are necessary for maintaining product quality, ensuring efficiency, and meeting regulatory standards. By carefully monitoring concentrations, industries can optimize their processes, reduce waste, and minimize environmental impact.
In research and development, concentrations are often used to understand the behavior and properties of substances. Scientists study how different concentrations of chemicals, enzymes, or other components affect reactions, solubility, and overall performance. These insights can then be used to guide the development of new materials, drugs, and technologies.
Overall, understanding concentrations in solutions is crucial across various disciplines. It allows for accurate measurements, appropriate dosing in medicine, efficient industrial processes, and valuable insights in research and development. Without this knowledge, it would be challenging to achieve accurate results, make informed decisions, and advance scientific understanding.
Factors that affect concentrations in solutions
In chemistry, the concentration of a solution refers to the amount of solute present in a given amount of solvent. Several factors can influence the concentration of a solution, including the amount and nature of the solute and solvent, as well as the temperature and pressure conditions.
Nature of the solute and solvent: The type of solute and solvent used can have a significant impact on the concentration of a solution. Different solutes have different abilities to dissolve in solvent, leading to variations in the concentration of the resulting solution. For example, a solute that is highly soluble in a particular solvent will result in a more concentrated solution compared to a solute with low solubility in the same solvent.
Amount of solute and solvent: The concentration of a solution is directly related to the amount of solute and solvent present. Increasing the amount of solute while keeping the amount of solvent constant will result in a higher concentration. Conversely, increasing the amount of solvent with a fixed amount of solute will lead to a lower concentration.
Temperature: Temperature can affect the solubility of a solute in a solvent, thereby influencing the concentration of a solution. In general, as the temperature increases, the solubility of most solutes also increases, leading to a higher concentration. However, there are exceptions to this rule, as some solutes may become less soluble at higher temperatures.
Pressure: Pressure changes can also impact the concentration of a solution, particularly when dealing with gases. Increasing the pressure on a gas solute can increase its solubility in a solvent, resulting in a higher concentration. Conversely, decreasing the pressure can lead to a lower concentration.
In summary, the concentration of a solution is influenced by several factors, including the nature of the solute and solvent, the amount of solute and solvent, the temperature, and the pressure. Understanding these factors is essential for controlling and manipulating the concentration of solutions in various chemical processes and applications.
Practical Applications of Concentrations in Solutions

In the field of chemistry, concentrations in solutions have various practical applications that are essential in different areas of research and industries. The ability to accurately measure and control concentrations allows scientists and engineers to achieve desired outcomes and meet specific requirements in their experiments and productions. Here are some practical applications:
1. Pharmaceutical Industry
In the pharmaceutical industry, concentrations in solutions play a crucial role in drug formulation and manufacturing. Developing the right concentration of active pharmaceutical ingredients (APIs) in medicines is essential for their effectiveness and safety. Precise control over concentrations ensures that drugs deliver the desired therapeutic effects without causing harmful side effects. Pharmaceutical companies also need to measure and control the concentrations of impurities and solvents in drug formulations to ensure product quality and comply with regulatory standards.
2. Environmental Monitoring
Concentrations in solutions are widely used in environmental monitoring to assess the pollution levels of air, water, and soil. Through techniques such as titration, spectroscopy, and chromatography, scientists can determine the concentrations of pollutants and contaminants in different environmental samples. This data is critical for assessing the impact of human activities on the environment, developing appropriate pollution control measures, and ensuring compliance with environmental regulations.
3. Food and Beverage Industry
In the food and beverage industry, concentrations in solutions are important for quality control, product development, and ensuring consumer safety. Measurements of concentrations are used to determine the levels of nutrients, additives, preservatives, and contaminants in food and beverage products. By maintaining precise concentrations, companies can produce consistent and safe products, as well as meet regulatory standards and customer expectations.
4. Chemical Manufacturing
Concentrations in solutions are critical in chemical manufacturing processes. Chemical reactions often require specific concentrations of reactants to achieve the desired reaction outcome and product yield. By accurately measuring and controlling concentrations, manufacturers can optimize reaction conditions, maximize product yield, minimize waste, and ensure the reproducibility of their processes. Concentration measurements are also important for quality control and monitoring the purity of chemical products.
Conclusion
Concentrations in solutions play a fundamental role in various industries and scientific research. Accurate measurement and control of concentrations are essential for achieving desired outcomes, ensuring product quality and safety, and complying with regulatory standards. The practical applications of concentrations in solutions span across pharmaceuticals, environmental monitoring, food and beverage, and chemical manufacturing, making concentrations a crucial parameter in countless processes and developments.