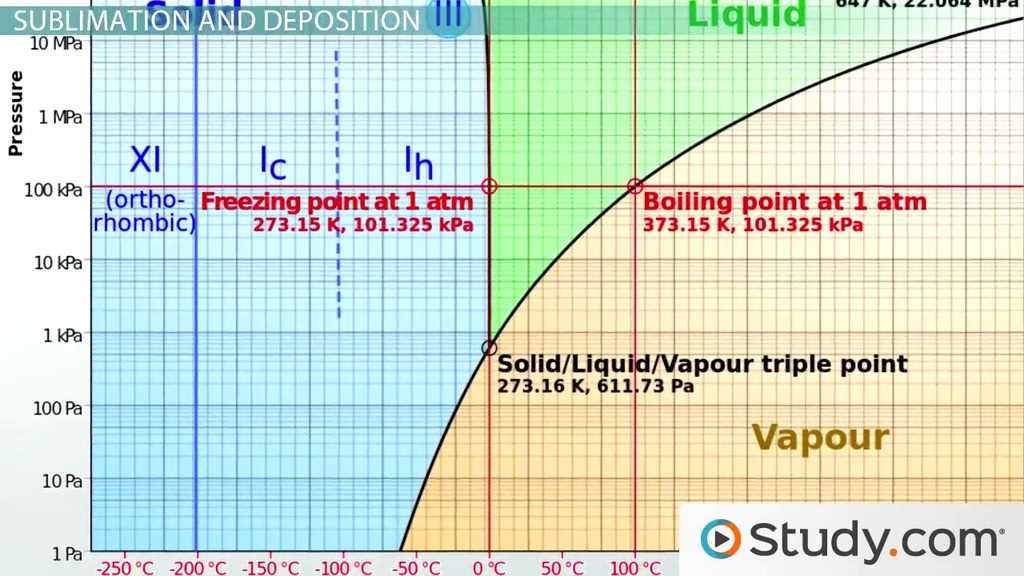
Thermodynamics is a branch of physics that deals with the relationships between heat and other forms of energy. It is a fundamental concept in the study of energy and its transformations. Understanding thermodynamics is crucial in many fields, including engineering, chemistry, and environmental science.
An answer key provides the correct answers to questions or problems posed in a textbook or test. It serves as a guide for students to check their work and ensure that they have understood the concepts correctly. In the context of thermodynamics, an answer key can be a valuable resource for students to verify their understanding of the subject.
The thermodynamics answer key contains solutions to various problems related to the laws of thermodynamics, heat transfer, and energy conversion processes. It typically includes step-by-step explanations and calculations to help students grasp the underlying principles and apply them in different scenarios.
By using the thermodynamics answer key, students can compare their answers with the correct ones and identify any misconceptions or errors they might have made. This allows them to learn from their mistakes and improve their understanding of thermodynamics. Additionally, the answer key can serve as a study aid, helping students practice and reinforce their knowledge of the subject.
Thermodynamics Answer Key: Your Comprehensive Guide
When it comes to studying thermodynamics, having a reliable answer key can make all the difference. A comprehensive answer key provides not only the correct solutions to problems but also a deeper understanding of the underlying concepts. With the right answer key, students can check their work, identify any mistakes, and learn from them.
One important aspect of a good answer key is its organization. It should be structured in a logical and easy-to-follow manner, with clearly labeled sections and sub-sections. This allows students to quickly find the specific problems they need help with, saving them valuable time and effort.
A high-quality answer key should also include detailed explanations for each solution. These explanations should not only provide the correct answer but also walk students through the steps and thought process involved in reaching it. This helps students understand the problem-solving techniques and principles used, empowering them to solve similar problems on their own in the future.
Additionally, a comprehensive answer key should cover a wide range of problem types and difficulty levels. This ensures that students have access to solutions for both basic and advanced thermodynamics concepts. It should also include examples that apply the principles of thermodynamics to real-world situations, helping students see the practical applications of the subject.
In conclusion, having a reliable and comprehensive answer key is essential for mastering thermodynamics. It provides students with a valuable resource to check their work, understand the underlying concepts, and improve their problem-solving skills. With the right answer key, students can confidently tackle thermodynamics problems and excel in their studies.
The Basics of Thermodynamics
Thermodynamics is the study of energy and its transformations. It is a branch of physics that deals with the relationships between heat, work, and energy. The principles of thermodynamics are essential in understanding how energy is transferred and converted in various systems, from simple engines to complex industrial processes.
One of the key concepts in thermodynamics is the idea of energy conservation. The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transferred or converted from one form to another. This principle forms the foundation of the study of thermodynamics and is crucial in analyzing and predicting the behavior of energy in different systems.
Temperature: Temperature is a measure of the average kinetic energy of the particles in a system. It is commonly measured in Celsius (°C) or Kelvin (K). In thermodynamics, temperature plays a crucial role in determining the direction of heat transfer between objects. Heat always flows from objects at higher temperatures to objects at lower temperatures until thermal equilibrium is reached.
Heat: Heat is the transfer of thermal energy between objects due to a temperature difference. It is a form of energy and is measured in joules (J) or calories (cal). Heat can be transferred through conduction (direct contact), convection (through fluid motion), or radiation (electromagnetic waves).
The Three Laws of Thermodynamics:
- The First Law (Law of Energy Conservation): States that the total energy of an isolated system is constant. Energy can neither be created nor destroyed, only transferred or converted from one form to another.
- The Second Law (Law of Entropy): States that the entropy (disorder) of an isolated system always increases over time. It indicates that natural processes tend to move towards a state of greater disorder.
- The Third Law (Law of Absolute Zero): States that the entropy of a system approaches a minimum value at absolute zero temperature (0 Kelvin or -273.15°C). It provides a fundamental basis for understanding the behavior of matter at very low temperatures.
Applications of Thermodynamics: Thermodynamics has numerous practical applications in various fields, including engineering, chemistry, biology, and environmental science. It is used in designing efficient engines, optimizing industrial processes, understanding chemical reactions and phase transitions, and assessing the energy efficiency of different systems.
In conclusion, thermodynamics is a fundamental branch of physics that deals with the study of energy and its transformations. It is based on the principles of energy conservation and entropy and has numerous practical applications in various scientific and engineering disciplines.
The Laws of Thermodynamics
The laws of thermodynamics are fundamental principles that govern the behavior of energy and matter in the universe. These laws provide a framework for understanding and predicting how energy moves and transforms in various systems. There are four laws of thermodynamics, each building upon the previous ones and contributing to our understanding of energy.
First Law: Energy Conservation
The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transferred or transformed from one form to another. This law helps us understand how energy is conserved within a system and how it can be transferred or converted between different forms, such as heat, work, and potential energy.
Second Law: Entropy
The second law of thermodynamics introduces the concept of entropy, which is a measure of the disorder or randomness in a system. It states that in any energy transfer or transformation, the total entropy of the system and its surroundings always increases. This law helps explain why certain processes are irreversible and why some forms of energy are less useful than others.
Third Law: Absolute Zero
The third law of thermodynamics states that it is impossible to reach absolute zero, the temperature at which all molecular motion stops, in a finite number of steps. This law provides a fundamental limit to how cold a system can become and establishes a reference point for temperature measurements.
Fourth Law: Nernst’s Theorem
The fourth law of thermodynamics, also known as Nernst’s theorem, deals with the behavior of an ideal system as it approaches absolute zero. It states that all processes in an ideal system at absolute zero will cease, and the entropy and enthalpy of the system will approach zero. This law helps us understand the behavior of matter and energy at extremely low temperatures.
In conclusion, the laws of thermodynamics are essential principles that guide our understanding of energy and its transformations. They provide a foundation for studying and predicting the behavior of various systems, from microscopic particles to astronomical bodies. These laws have profound implications in various scientific and engineering fields and continue to shape our understanding of the physical world.
Key Vocabulary and Definitions
In the study of thermodynamics, it is essential to have a solid understanding of the key vocabulary and definitions associated with the subject. The following are some of the important terms:
1. Thermodynamics:
Thermodynamics is the branch of physics that deals with the relationships between heat, work, and energy. It encompasses the principles governing the behavior of systems in equilibrium and non-equilibrium states.
2. System:
A system refers to the specific portion of the universe being studied. It can be a physical object, such as a gas in a container, or it can be an abstract concept, such as a thermodynamic process.
3. Surroundings:
The surroundings, also known as the environment, are everything outside the system under consideration. It can include other objects, the atmosphere, or even the entire universe.
4. Heat:
Heat is a form of energy that is transferred between two objects or systems due to a temperature difference. It flows from a higher temperature to a lower temperature until thermal equilibrium is reached.
5. Work:
In the context of thermodynamics, work refers to the energy transferred to or from a system due to the application of a force through a displacement. It can be expressed as the product of the force applied and the distance over which the force is exerted.
6. Internal Energy:
Internal energy is the sum of all the microscopic energy stored within a system. It includes the kinetic energy of the molecules, the potential energy of their intermolecular forces, and any other forms of energy associated with the system.
7. First Law of Thermodynamics:
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed but can only be converted from one form to another or transferred between systems and their surroundings.
8. Second Law of Thermodynamics:
The second law of thermodynamics states that the entropy of an isolated system always increases over time. It provides a direction for spontaneous processes and explains why certain processes are irreversible.
9. Entropy:
Entropy is a measure of the randomness or disorder in a system. It quantifies the number of possible microstates corresponding to a given macrostate and is related to the probability of a system being in a particular state.
10. Equilibrium:
Equilibrium refers to a state of balance in which there is no net change or transfer of energy between a system and its surroundings. It can be thermal, mechanical, or chemical in nature.
These are just a few of the key vocabulary terms and definitions used in thermodynamics. Familiarizing yourself with these concepts will provide a solid foundation for understanding the principles and laws governing the behavior of energy and systems.
Thermodynamic Systems and Processes
A thermodynamic system is a region in space that is under study. It can be as small as a single molecule or as large as the entire universe. A system is defined by its boundaries, which could be fixed or moving. The boundaries of a system can be real or imaginary, but they are used to separate the system from its surroundings. The surroundings include everything outside the boundaries of the system.
There are three types of thermodynamic systems: isolated, closed, and open. An isolated system does not exchange energy or matter with its surroundings. A closed system allows energy, but not matter, to cross its boundaries. An open system allows both energy and matter to cross its boundaries.
A thermodynamic process is a change in the state of a system. It can occur through various interactions between the system and its surroundings, such as heat transfer and work done on or by the system. The behavior of a system during a process can be analyzed using different thermodynamic properties, such as temperature, pressure, and volume.
There are different types of thermodynamic processes, including isothermal, adiabatic, isobaric, and isochoric processes. An isothermal process occurs at constant temperature, while an adiabatic process occurs without any heat transfer between the system and its surroundings. During an isobaric process, the pressure remains constant, and during an isochoric process, the volume remains constant.
In summary, thermodynamic systems and processes play a fundamental role in understanding and analyzing the behavior of physical systems. By studying different types of systems and processes, scientists and engineers can design and optimize various technological applications, such as engines, refrigerators, and power plants.
Applications of Thermodynamics
Thermodynamics is a branch of physics that deals with the study of heat, work, and energy. It has a wide range of applications in various fields, including engineering, chemistry, biology, and environmental science. Understanding thermodynamics is essential for designing efficient systems and processes.
Power Generation: One of the most significant applications of thermodynamics is in power generation. Thermodynamic principles are used to design and optimize power plants, such as steam turbines, gas turbines, and internal combustion engines. The conversion of heat into mechanical work relies on thermodynamic cycles like the Rankine cycle and the Carnot cycle.
Heat Transfer: Thermodynamics plays a crucial role in understanding and controlling heat transfer processes. It helps in the design of efficient heat exchangers for applications like air conditioning, refrigeration, and industrial processes. Thermodynamic principles are also applied in the analysis of heat transfer in materials, such as determining the thermal conductivity of a substance.
Chemical Reactions: Thermodynamics is used to study and predict the behavior of chemical reactions. It helps in determining the feasibility of a reaction and calculating important quantities like enthalpy, entropy, and Gibbs free energy. Thermodynamic principles are essential for designing chemical processes, such as the production of fertilizers, pharmaceuticals, and polymers.
Environmental Science: Thermodynamics is crucial in understanding and addressing environmental issues. It is used to study and model the behavior of pollutants in the environment, such as the movement and transformation of contaminants in soil and water systems. Thermodynamic principles also play a role in analyzing and optimizing renewable energy systems, such as solar cells and fuel cells.
Biology: Thermodynamic principles are applicable in biological systems, particularly in the study of metabolism and energy conversion. Understanding the thermodynamics of biological processes helps in analyzing and optimizing metabolic pathways, such as the production of energy-rich molecules like ATP. It also provides insights into the efficiency and regulation of biological systems.
In summary, thermodynamics has numerous applications in different fields, ranging from power generation and heat transfer to chemical reactions, environmental science, and biology. Its principles and concepts are crucial for designing efficient systems, predicting behavior, and optimizing processes.
Common Problems and Solutions
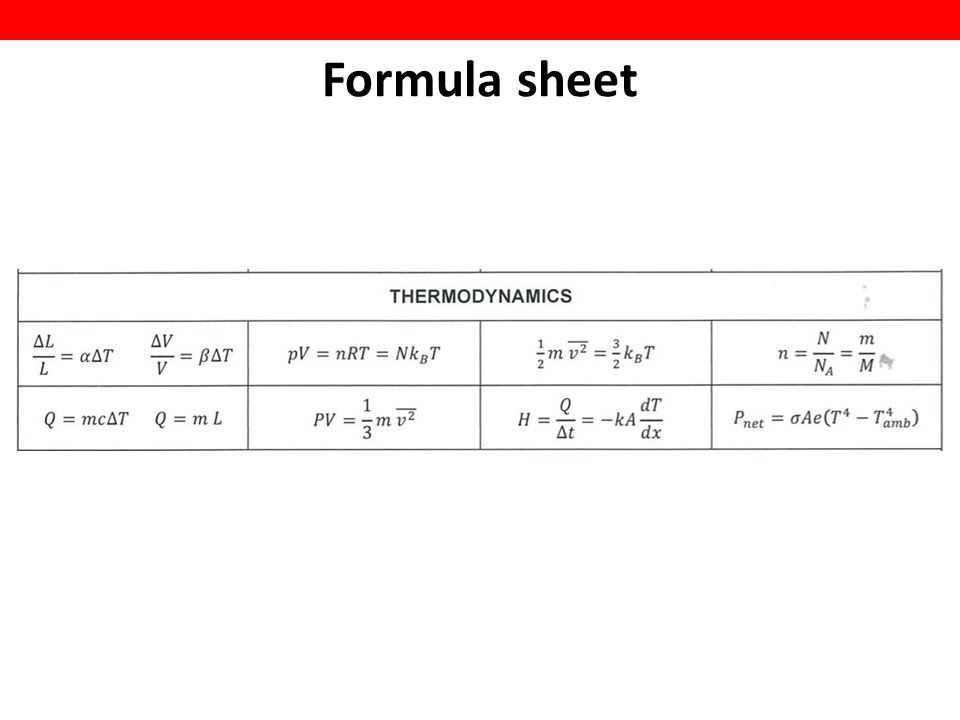
In the study of thermodynamics, there are several common problems that students may encounter. These problems can be challenging, but with a clear understanding of the underlying concepts and some problem-solving techniques, they can be effectively solved. Here are some of the common problems and their solutions:
-
Problem: Calculating the work done in a thermodynamic process.
Solution: To calculate the work done, one can use the formula W = PΔV, where W is the work done, P is the pressure, and ΔV is the change in volume. By substituting the given values into the equation and solving for W, the work done can be determined.
-
Problem: Determining the efficiency of a heat engine.
Solution: To calculate the efficiency of a heat engine, one can use the formula η = 1 – (Tc/Th), where η is the efficiency, Tc is the temperature of the cold reservoir, and Th is the temperature of the hot reservoir. By plugging in the values and performing the calculation, the efficiency of the heat engine can be determined.
-
Problem: Calculating the change in entropy.
Solution: To calculate the change in entropy, one can use the formula ΔS = Q/T, where ΔS is the change in entropy, Q is the heat added or removed, and T is the temperature. By substituting the given values into the equation, the change in entropy can be calculated.
These are just a few examples of common problems in thermodynamics. It is important to carefully read and understand the problem statement, identify the relevant concepts and equations, and apply the appropriate problem-solving techniques. By practicing and gaining familiarity with these techniques, one can effectively solve thermodynamics problems and gain a deeper understanding of the subject.