Welcome to the highly anticipated Sweet 16 Chemistry of Gases Tournament! This electrifying event is where the best and brightest chemists come together to showcase their knowledge of gases and battle it out for the coveted title of champion. In this article, we will reveal the answer key to the most challenging questions from the tournament and explore the fascinating world of gases.
Gas chemistry plays a crucial role in various scientific and everyday applications. Understanding the properties, behaviors, and interactions of gases is essential for fields such as environmental science, engineering, medicine, and more. During the Sweet 16 Chemistry of Gases Tournament, participants were tested on their knowledge of gas laws, stoichiometry, kinetic theory, and other fundamental concepts.
In this article, we will dive into the answers to some of the most intriguing questions asked during the tournament. Gas laws, such as Boyle’s Law and Charles’s Law, were put to the test with complex scenarios and equations. Participants were also challenged to calculate the molar mass of gases and determine their behavior under various conditions.
Sweet 16 Chemistry of Gases Tournament Answer Key
The Sweet 16 Chemistry of Gases Tournament has come to a thrilling conclusion, and we have the answer key to reveal the winners! Over the past few weeks, students from all over the country have been competing to showcase their knowledge of the properties and behavior of gases. Now it’s time to see who came out on top!
The tournament consisted of a series of challenging questions and scenarios that tested the participants’ understanding of concepts such as gas laws, stoichiometry, and the behavior of gases in different conditions. The answers were scored based on accuracy, thoroughness, and clarity of explanation. Without further ado, let’s announce the winners of each round!
Round 1: Boyle’s Law Battle

- Winner: Team Avogadro’s Avengers
- Runner-Up: Team Dalton’s Disciples
In this round, teams were asked to solve problems involving Boyle’s Law, which describes the relationship between the pressure and volume of a gas at constant temperature. Team Avogadro’s Avengers emerged victorious with their quick problem-solving skills and clear explanations.
Round 2: Charles’s Law Challenge
- Winner: Team Gay-Lussac’s Gases
- Runner-Up: Team Lavoisier’s Legacy
Charles’s Law, which relates the volume and temperature of a gas at constant pressure, was the focus of this round. Team Gay-Lussac’s Gases impressed the judges with their detailed calculations and thorough understanding of the concept.
Round 3: Ideal Gas Showdown

- Winner: Team Boyle’s Besties
- Runner-Up: Team Avogadro’s Avengers
This round tested the participants’ knowledge of the ideal gas law, which combines Boyle’s Law, Charles’s Law, and Avogadro’s Law. Team Boyle’s Besties emerged as the winners, showcasing their mastery of all three gas laws and their ability to apply them in various scenarios.
Round 4: Gas Stoichiometry Slam
- Winner: Team Dalton’s Disciples
- Runner-Up: Team Lavoisier’s Legacy
Gas stoichiometry was the focus of this intense round, where teams had to balance chemical equations and calculate the quantities of reactants and products. Team Dalton’s Disciples proved their expertise in this area, providing accurate calculations and logical explanations.
Congratulations to all the winners for their outstanding performance in the Sweet 16 Chemistry of Gases Tournament! It was a fierce competition, and all the participants should be proud of their hard work and dedication to mastering the chemistry of gases.
Tournament Overview
The Sweet 16 Chemistry of Gases Tournament showcased the knowledge and skills of high school students from across the country in the field of chemistry. The tournament, organized by the National Chemistry Association, consisted of a series of challenging questions and hands-on experiments that tested the participants’ understanding of the properties and behavior of gases.
During the tournament, students were divided into teams of four and competed against each other in a bracket-style format. The teams were given a set of questions and had a limited amount of time to provide the correct answers. The questions covered a wide range of topics, including gas laws, stoichiometry, and the behavior of gases in different scenarios.
The tournament also included a practical component, where teams were required to perform experiments related to gases. These experiments were designed to assess the participants’ ability to apply their theoretical knowledge to real-world scenarios. The teams were evaluated based on their experimental procedure, data collection and analysis, and the accuracy of their results.
The Sweet 16 Chemistry of Gases Tournament provided an opportunity for students to showcase their passion for chemistry and demonstrate their ability to apply scientific principles to solve complex problems. The competition was not only a test of knowledge, but also a platform for students to learn from each other and exchange ideas. It served as a reminder of the importance of chemistry in our everyday lives and the impact it has on the world around us.
Tournament Format
The Sweet 16 Chemistry of Gases Tournament is a unique competition that tests the knowledge and skills of chemistry students in the study of gases. The tournament follows a rigorous format to ensure fairness and to showcase the participants’ understanding of this topic.
The tournament begins with a preliminary round where all the participating students take a written exam. This exam assesses their knowledge of gas laws, the behavior of gases, and other related concepts. The top 16 scorers from this round advance to the Sweet 16 round, where the actual tournament takes place.
Sweet 16 Round
The Sweet 16 round consists of a series of head-to-head matches between the remaining participants. Each match comprises a set of questions and problems that the students must solve within a given time limit. These questions and problems are designed to challenge the students’ understanding of gas laws and their ability to apply them to real-world scenarios.
Each match is overseen by a panel of judges, including experienced chemistry teachers and experts in the field. The judges evaluate the students’ responses and award points based on the accuracy and clarity of their answers. The student with the highest score at the end of each match advances to the next round, while the other student is eliminated from the tournament.
The matches continue in a single-elimination format until only two students remain for the championship match. The championship match is a high-stakes battle where the two finalists compete to determine the ultimate winner of the tournament. The student who emerges victorious in this match is crowned the champion of the Sweet 16 Chemistry of Gases Tournament.
The tournament format ensures that only the most knowledgeable and skilled students advance to the later rounds. It provides a platform for students to showcase their understanding of gas laws and their ability to apply them in practical situations. The Sweet 16 Chemistry of Gases Tournament is a thrilling competition that promotes the study of chemistry and recognizes the achievements of outstanding young chemists.
Tournament Questions
In the Sweet 16 chemistry of gases tournament, teams of students compete to answer questions about the properties and behavior of gases. The tournament questions cover topics such as gas laws, gas pressure, gas stoichiometry, and the behavior of gases in different conditions.
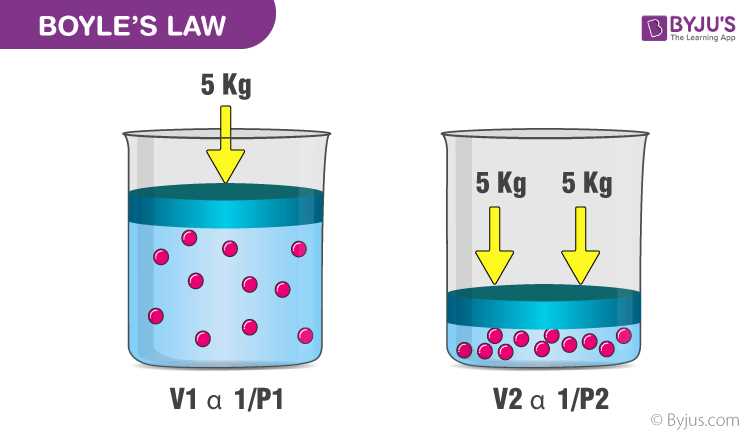
One of the tournament questions asks students to identify the gas law that describes the relationship between the pressure and volume of a gas at constant temperature. The correct answer is Boyle’s Law, which states that the pressure of a gas is inversely proportional to its volume when temperature is held constant.
Another tournament question asks students to calculate the molar mass of a gas given the mass of the gas and its volume at a certain temperature and pressure. This question tests students’ understanding of the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas.
Additional tournament questions may require students to compare the behavior of different gases at different temperatures and pressures, explain the factors that affect gas solubility, or solve problems involving gas stoichiometry. These questions challenge students to apply their knowledge of gas laws and principles to real-world scenarios.
The Sweet 16 chemistry of gases tournament questions are designed to test students’ understanding of the properties and behavior of gases and to encourage critical thinking and problem-solving skills. By competing in the tournament, students have the opportunity to deepen their understanding of gas chemistry and to apply their knowledge in a competitive setting.
Answer Key

In the Sweet 16 chemistry of gases tournament, participants were challenged to answer a series of questions related to the properties and behavior of gases. The answer key provides the correct answers to these questions and serves as a reference for participants to check their answers. Here are the correct answers for each question:
Question 1: Which gas is the most abundant in Earth’s atmosphere?
Answer: Nitrogen
Question 2: What is the SI unit of pressure?
Answer: Pascal
Question 3: What is the ideal gas law equation?
Answer: PV = nRT
Question 4: What happens to the volume of a gas if its temperature remains constant but the pressure increases?
Answer: The volume decreases
Question 5: What is the molar mass of oxygen gas (O2)?
Answer: 32 g/mol
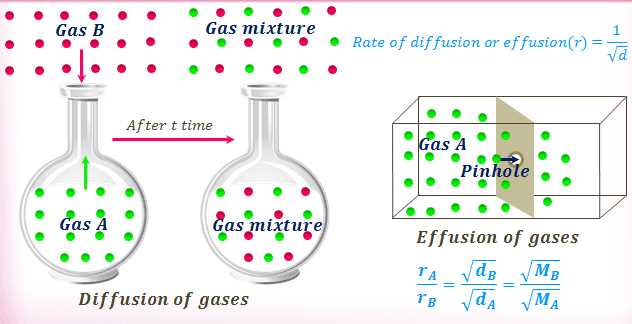
Question 6: What is the process of gas particles spreading out and mixing with each other called?
Answer: Diffusion
Question 7: When does a gas deviate most from ideal behavior?
Answer: At high pressures and low temperatures
Question 8: What is the mass of 2 moles of helium gas (He)?
Answer: 8 grams
Question 9: What is the boiling point of water in Celsius?
Answer: 100°C
Question 10: What is the relationship between pressure and volume, when the temperature and amount of gas are held constant?
Answer: They are inversely proportional (Boyle’s Law)
Participants can use this answer key to verify their answers and assess their understanding of the concepts covered in the tournament. It is important to review the explanations provided for each correct answer and address any misconceptions to further enhance their knowledge of the chemistry of gases.
Scoring
Throughout the Sweet 16 chemistry of gases tournament, the participating teams competed fiercely, showcasing their knowledge and understanding of the chemistry of gases. The scoring system was designed to reward teams based on their accurate and timely answers to the tournament questions.
Each correct answer was awarded one point, while incorrect or incomplete answers received no points. In addition, teams were given the opportunity to earn bonus points through various challenges, such as solving additional chemistry problems or providing detailed explanations for their answers.
The scoring system also took into account the speed at which teams submitted their answers. In cases where two or more teams provided equally accurate answers, the team that submitted their response first was awarded an extra point as a tiebreaker. This added an element of excitement and urgency to the tournament, encouraging teams to respond quickly and accurately in order to gain an advantage.
At the end of the tournament, the team with the highest cumulative score was declared the winner. Their exceptional knowledge and performance throughout the tournament showcased their mastery of the chemistry of gases. All participating teams demonstrated their dedication, teamwork, and passion for chemistry, making the Sweet 16 chemistry of gases tournament a memorable and rewarding experience for all.