Accuracy and precision are two important concepts in measurement and data analysis. They are often used in scientific experiments and research to evaluate the reliability and validity of the results obtained. Understanding the difference between accuracy and precision is crucial for researchers and scientists to ensure the quality of their work.
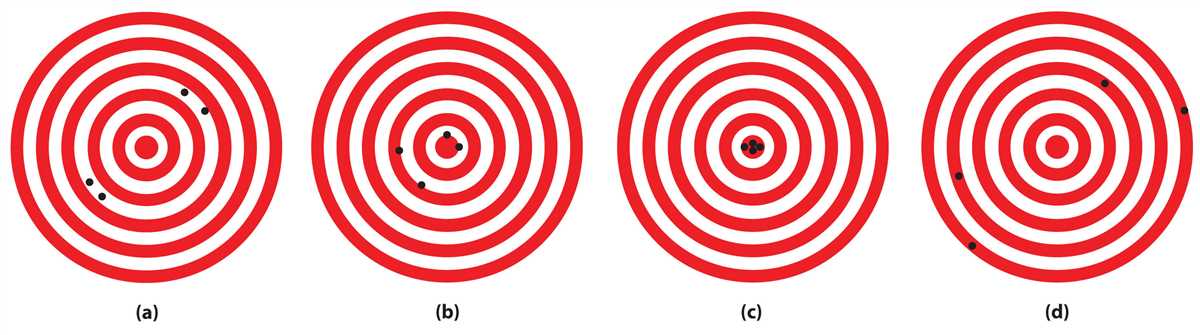
Accuracy refers to how close a measured value is to the true or accepted value. In other words, it measures how well the data reflects the real world. An accurate measurement is one that has a small systematic error, meaning that it is not influenced by any biases or systematic deviations from the true value. Achieving accuracy requires careful calibration of instruments and careful elimination of any sources of error.
Precision, on the other hand, refers to the repeatability or consistency of a measured value. It measures how close multiple measurements are to each other. A precise measurement is one that has a small random error, meaning that the measurements are consistent and reproducible. Precision can be improved by taking multiple measurements and calculating the average, as this reduces the impact of random fluctuations and errors.
Accuracy and Precision Worksheet Answers: Mastering the Basics
In the field of science, accuracy and precision are two crucial concepts that help researchers and scientists make reliable and valid conclusions based on their observations and measurements. Accuracy refers to how close a measurement or result is to the true or accepted value. Precision, on the other hand, refers to how close the measurements or results are to each other.
When working on an accuracy and precision worksheet, it is important to understand the basic principles behind these concepts. The answers to the worksheet will help you master the basics and improve your overall understanding.
- Accuracy: The key to achieving accuracy is avoiding systematic errors and minimizing random errors. In the worksheet, you may come across questions that ask you to identify sources of error and suggest ways to minimize them. These answers will help you develop a critical eye when it comes to evaluating the accuracy of your measurements.
- Precision: Precision is all about consistency and reproducibility. In the worksheet, you might encounter questions that require you to calculate the standard deviation or determine the number of significant figures in a measurement. These answers will guide you in understanding how precise your measurements are and how to express them appropriately.
- Uncertainty: Another important aspect of accuracy and precision is uncertainty. This refers to the range of possible values that a measurement or result may fall within. The worksheet may include questions that ask you to calculate the percent uncertainty or determine the minimum and maximum possible values. These answers will help you better understand the limitations and potential errors in your measurements.
Overall, by mastering the basics of accuracy and precision and having a solid understanding of the concepts, you will be better equipped to analyze and interpret scientific data effectively. The worksheet answers provide valuable insights and explanations that will enhance your scientific skills and help you become a more proficient researcher or scientist.
The Importance of Understanding Accuracy and Precision
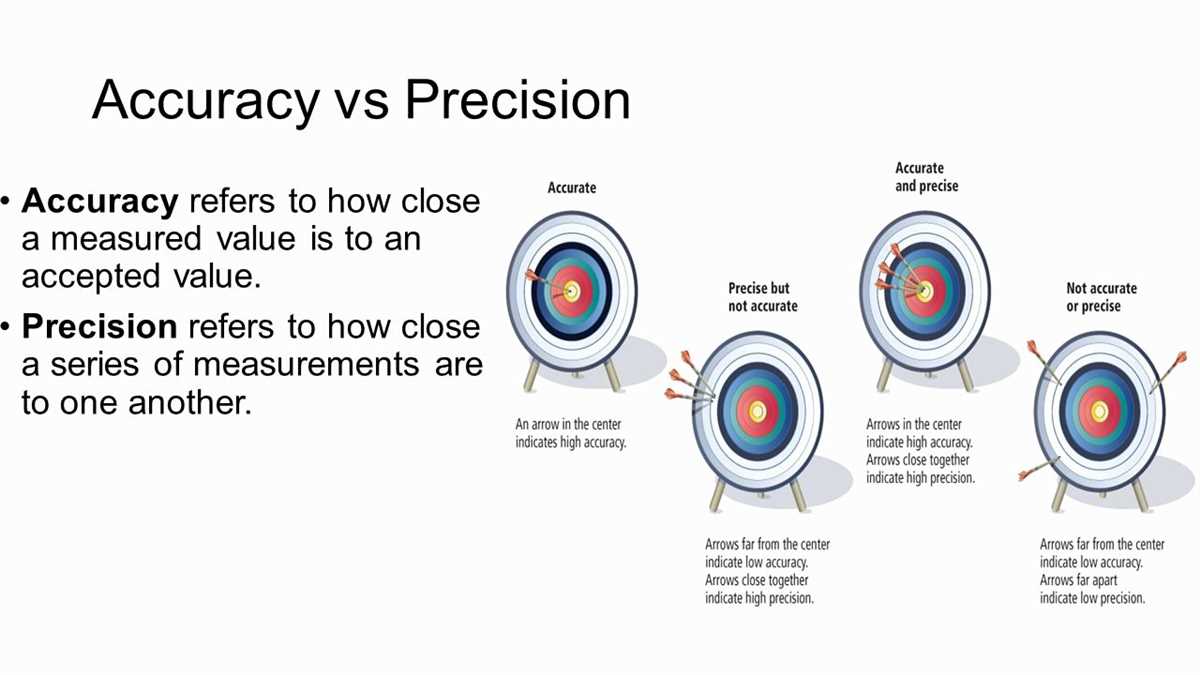
For scientists, researchers, and anyone working with data, understanding accuracy and precision is vital. Accuracy refers to how close a measurement is to the true value, while precision refers to how close measurements are to each other. These concepts play a crucial role in ensuring the reliability and validity of experimental results.
Accuracy: To obtain accurate results, it is important to minimize systematic errors. Systematic errors are consistent and repeatable errors that affect the accuracy of measurements. By understanding accuracy, researchers can identify and correct for these errors, ensuring that their measurements are as close to the true value as possible.
Precision: Precision is equally important as accuracy in scientific measurements. Precision reflects the repeatability and consistency of measurements. A high precision indicates that the measurements are close to each other, making the results more reliable. On the other hand, low precision indicates inconsistency and variability of measurements, which can lead to unreliable results.
To achieve accurate and precise measurements, scientists use various techniques and instruments. These may include calibrating instruments, improving experimental procedures, and using statistical analysis methods to identify outliers and improve data accuracy. Understanding accuracy and precision helps researchers to evaluate the quality of their data and make informed decisions based on the results.
Uncertainty: Accuracy and precision are closely related to uncertainty. Uncertainty quantifies the confidence or range of possible values for a measurement. By understanding accuracy and precision, researchers can estimate and account for the uncertainty associated with their measurements, making their conclusions more robust and reliable.
In conclusion, having a thorough understanding of accuracy and precision is crucial for anyone working with data. These concepts help ensure the reliability and validity of measurements and enable researchers to make accurate and informed conclusions. By incorporating accurate and precise measurements into scientific investigations, we can enhance our understanding of the world around us and make meaningful contributions to scientific knowledge.
Defining Accuracy: The Measure of Agreement with the True Value
The concept of accuracy is fundamental in scientific measurements as it represents the level of agreement between an experimental result and the true value. Accuracy is a measure of how close a result is to the accepted or known value. In other words, it measures the correctness or trueness of a measurement.
To determine the accuracy of a measurement, it is necessary to compare the measured value with the true value. The true value is often obtained through a reference standard or a known value established by an authoritative source. For example, if we were measuring the length of an object using a ruler, the true value would be the actual length of the object, which can be obtained through more accurate measurement methods or by consulting a reliable source.
The accuracy of a measurement can be described in terms of the percent error. Percent error is a way to express the difference between the measured value and the true value as a percentage of the true value. It is calculated using the formula:
Percent error = [(Measured value – True value) / True value] * 100%
For instance, if the true value of an object’s length is 10 cm and the measured value using a ruler is 9.5 cm, the percent error would be calculated as follows:
- Measured value – True value = 9.5 cm – 10 cm = -0.5 cm
- (-0.5 cm / 10 cm) * 100% = -5%
The negative sign indicates that the measured value is lower than the true value, while the magnitude represents the percentage difference. In this example, the percent error is -5%, indicating that the measurement is 5% lower than the true value.
It is important to note that accuracy and precision are distinct terms. Accuracy refers to the agreement with the true value, while precision refers to the consistency or reproducibility of measurements. A measurement can be precise without being accurate (showing consistent results that are far from the true value), and it can be accurate without being precise (showing results that are close to the true value, but with large variations between measurements).
In summary, accuracy is an essential quality of scientific measurements as it indicates how closely a result aligns with the true value. It is determined by comparing the measured value with the true value, usually expressed as a percentage error. By evaluating accuracy, researchers can assess the reliability and validity of their measurements and ensure the quality of their experimental data.
Exploring Precision: The Measure of Consistency and Reproducibility
In scientific experiments and measurements, precision plays a crucial role in determining the reliability and accuracy of the results. Precision refers to the measure of consistency and reproducibility of a given set of data. It quantifies the degree to which repeated measurements or trials will yield similar results, providing an indication of the reliability of the data.
Precision is often described as the ability to obtain consistent and closely-clustered results. For example, in a laboratory setting, if multiple measurements of a substance’s melting point yield similar values, it indicates high precision. Conversely, if the measurements vary significantly from each other, it suggests low precision. Scientists strive for high precision to reduce uncertainty and increase the confidence in the accuracy of their findings.
To quantify precision, statistical measures such as standard deviation and coefficient of variation are commonly used. Standard deviation calculates the average deviation from the mean, indicating the spread of the data. A low standard deviation indicates high precision, while a high standard deviation suggests low precision. Coefficient of variation expresses the standard deviation as a percentage of the mean, allowing for comparison of precision across different datasets or experiments.
In addition to the statistical measures, precision can also be assessed visually using graphical representations such as error bars and scatterplots. Error bars, represented as lines around data points, indicate the range of variability in the measurements. The narrower the error bars, the higher the precision. Scatterplots, on the other hand, visually depict the distribution of data points, with a tighter cluster suggesting higher precision.
By ensuring precision in measurements and experiments, scientists enhance the reliability and reproducibility of their findings. It allows for more accurate comparisons between different studies, facilitates the identification of trends and patterns, and increases confidence in the validity of the results. Ultimately, precision is a critical factor in scientific research and plays a vital role in advancing knowledge and understanding in various fields.
Calculating Accuracy and Precision: Worksheet Exercises and Solutions
The concept of accuracy and precision is important in scientific measurements. Accuracy refers to how close a measured value is to the true value, while precision refers to how closely multiple measurements of the same quantity agree with each other. To develop a better understanding of these concepts, students often complete worksheet exercises that involve calculating accuracy and precision.
These worksheet exercises typically present students with a set of data points and ask them to determine the accuracy and precision of the measurements. Students are required to calculate the average or mean of the data points and compare it to the accepted or true value. The difference between the average and the accepted value is used to determine the accuracy of the measurements.
In addition to determining accuracy, students also calculate the standard deviation of the data points to determine the precision of the measurements. The standard deviation measures the spread or variability of the data points around the mean. A smaller standard deviation indicates higher precision, while a larger standard deviation indicates lower precision.
Worksheet exercises often include solutions or answer keys that provide step-by-step explanations of how to calculate accuracy and precision. These solutions help students understand the process and improve their problem-solving skills. Additionally, the solutions may include discussion questions or additional exercises to further reinforce the concepts of accuracy and precision.
By completing these worksheet exercises and understanding the solutions, students can improve their ability to accurately and precisely measure quantities in scientific experiments and investigations. This mastery of accuracy and precision is essential for the development of scientific skills and the generation of reliable data.
Common Sources of Error: Understanding and Minimizing Variability
When conducting experiments or measurements, it is crucial to understand and minimize variability in order to obtain accurate and precise results. Variability refers to the differences or fluctuations that can occur within a set of data, and it can arise from various sources of error. By identifying and addressing these sources of error, researchers can improve the reliability and validity of their findings.
1. Instrumentation Error:
One common source of error is instrumentation error, which is caused by inaccuracies or limitations in the tools used for measurement. This can include issues such as imprecise scales, faulty sensors, or calibration errors. To minimize this type of error, it is important to regularly calibrate, maintain, and validate the instruments used in the experiment. Additionally, using multiple instruments or repeating measurements can help identify and reduce instrumentation error.
2. Human Error:
Human error can also contribute to variability in experimental results. This can occur due to factors such as improper technique, biased observations, or inconsistent execution of procedures. To minimize human error, researchers can provide clear instructions and training to those conducting the experiment. It is also important to have multiple researchers independently perform the measurements or observations to check for consistency and mitigate the impact of individual errors.
3. Environmental Conditions:
The environment in which an experiment takes place can introduce variability in the measurements. Factors such as temperature, humidity, and atmospheric pressure can all affect the outcomes. To minimize the impact of environmental conditions, experiments should be conducted in controlled settings or under controlled conditions. This can include using environmental chambers, maintaining stable temperature and humidity levels, or making necessary adjustments and corrections based on environmental factors.
4. Sample Size and Selection:
The size and selection of the sample being studied can also contribute to variability. A small sample size may not adequately represent the larger population, leading to inaccurate conclusions. Similarly, biased or non-random selection of samples can introduce errors. To mitigate these issues, researchers should aim for large and representative sample sizes. Randomization and proper sampling techniques should also be employed to ensure unbiased results.
By understanding and minimizing these common sources of error, researchers can improve the reliability and validity of their experimental results. Attention to detail, rigorous protocols, and careful consideration of potential sources of variability are essential for obtaining accurate and precise data in scientific investigations.
Improving Accuracy and Precision in Scientific Measurements: Best Practices
In order to obtain reliable and meaningful data, it is essential for scientists to strive for both accuracy and precision in their measurements. Here are some best practices that can help improve the accuracy and precision of scientific measurements:
1. Calibration
Regularly calibrating measuring instruments is crucial for obtaining accurate measurements. Calibrating instruments ensures that they are reading correctly and are free from any systematic errors. It is important to follow the manufacturer’s instructions for calibration and perform the procedure as recommended.
2. Standardization
Using standardized materials or methods can improve the accuracy and precision of measurements. By using known standards, such as certified reference materials, scientists can compare their measurements against a known value to ensure accuracy. Similarly, using standardized protocols and techniques can reduce variability and improve precision among different measurements.
3. Repetition
Repeating measurements multiple times can help identify and eliminate random errors, thus improving precision. By taking multiple readings and calculating the average, scientists can reduce the impact of random fluctuations and obtain a more precise measurement.
4. Environmental Control
Environmental factors, such as temperature, humidity, and air pressure, can influence the accuracy and precision of measurements. It is important to control and monitor these variables to minimize their effects. Working in a controlled laboratory environment with stable conditions can help improve the reliability of measurements.
5. Proper Technique
Using proper techniques and following best practices for handling and operating measuring instruments is essential for accurate and precise measurements. This includes ensuring proper alignment, minimizing parallax error, using appropriate units, and documenting all relevant information.
By implementing these best practices, scientists can significantly improve the accuracy and precision of their measurements, leading to a better understanding of the phenomena under study and more reliable scientific conclusions.