
Welcome to the answer key for Chapter 3 of “Matter and Change”. In this chapter, we will be exploring the fundamental concepts of matter and its properties, as well as the changes it can undergo. Understanding these concepts is crucial in the study of chemistry, as they lay the foundation for more advanced topics and applications.
In the previous chapters, we learned that matter is anything that has mass and occupies space. In Chapter 3, we will delve deeper into the properties of matter, such as its physical and chemical properties. Physical properties are characteristics that can be observed or measured without changing the identity of the substance, while chemical properties describe how matter behaves during chemical reactions or interactions with other substances.
Throughout this chapter, you will encounter various types of changes that matter can undergo. Physical changes refer to alterations in the physical properties of a substance, such as changes in state, shape, or size. Chemical changes, on the other hand, involve the reorganization of atoms and the formation of new substances.
By the end of this chapter, you should have a solid understanding of the different properties of matter and the changes it can undergo. With the help of this answer key, you will be able to check your understanding of the concepts presented in the chapter and ensure that you have grasped the key principles. So let’s dive in and explore the fascinating world of matter and change!
Understanding Matter and Change: Chapter 3 Answer Key
In Chapter 3 of the “Understanding Matter and Change” textbook, students are introduced to various key concepts and principles related to matter and its changes. This answer key provides an invaluable resource for students to check their understanding and comprehension of the chapter’s content.
The answer key covers a wide range of topics, including the classification of matter, physical and chemical properties, changes in matter, and the conservation of mass and energy. Each question in the chapter is carefully analyzed and answered in a clear and concise manner, helping students grasp the fundamental principles of matter and change.
- The first section of the answer key focuses on the classification of matter, distinguishing between pure substances and mixtures. It explains the difference between elements and compounds, and provides examples to illustrate these concepts.
- The second section discusses physical and chemical properties of matter. Students learn how to identify and describe these properties, and understand the relationship between properties and changes in matter.
- The third section explores different types of changes in matter, including physical changes such as phase changes and chemical changes such as reactions. The answer key provides step-by-step explanations for these changes, making it easier for students to understand the underlying processes.
- The final section of the answer key focuses on the conservation of mass and energy. Students learn about the law of conservation of mass and the law of conservation of energy, and how these laws apply to various situations involving matter and change.
Overall, the “Understanding Matter and Change: Chapter 3 Answer Key” is a valuable tool for students to reinforce their understanding of the chapter’s content and assess their knowledge. It serves as a comprehensive guide, helping students grasp the complex concepts and principles of matter and change with clarity and confidence.
Properties of Matter
Matter is anything that has mass and takes up space. It can exist in three different states: solid, liquid, or gas. Each state of matter has its own unique properties that define its behavior and characteristics.
Physical properties are characteristics of matter that can be observed or measured without changing the substance’s identity. These properties include color, shape, size, density, and melting point. For example, the physical property of boiling point refers to the temperature at which a substance changes from a liquid to a gas.
Chemical properties, on the other hand, describe the ability of a substance to undergo a chemical reaction and form new substances with different properties. Chemical properties are often observed when a substance reacts with another substance, such as its ability to burn or react with acid. These properties are related to the substance’s composition and structure.
The properties of matter can also be categorized as extensive or intensive. Extensive properties depend on the amount of matter present, such as mass or volume. Intensive properties, on the other hand, are independent of the amount of matter and remain the same regardless of the quantity. Examples of intensive properties include boiling point, density, and color.
In summary, matter has both physical and chemical properties that define its behavior and characteristics. Understanding these properties is essential for studying and manipulating different substances in various fields like chemistry and physics.
States of Matter

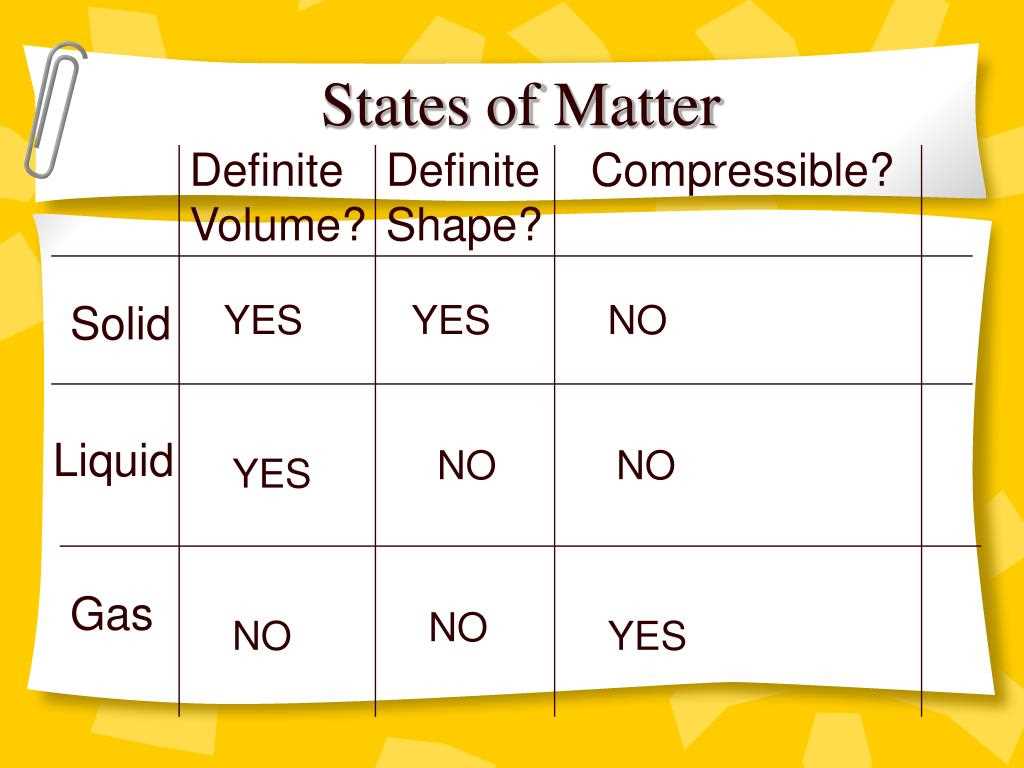
In the study of matter and change, it is important to understand the different states of matter that exist in our world. Matter can exist in three primary states: solid, liquid, and gas. Each state has its own unique characteristics and properties.
A solid is a state of matter that has a definite shape and volume. The particles in a solid are tightly packed together and have a fixed position. They vibrate in place but do not move freely. Solids have strong intermolecular forces which give them their definite shape and volume. Examples of solids include ice, wood, and metal.
A liquid is a state of matter that has a definite volume but takes the shape of its container. In a liquid, the particles are close together but can move past each other, allowing the liquid to flow. Liquids have weaker intermolecular forces compared to solids, which is why they do not have a definite shape. Examples of liquids include water, oil, and milk.
A gas is a state of matter that has neither a definite shape nor volume. Gas particles are far apart and move freely. They have weak intermolecular forces and are able to fill any container they are placed in. Gases can be compressed and expanded easily. Examples of gases include oxygen, nitrogen, and carbon dioxide.
Understanding the different states of matter is crucial in many areas of science and everyday life. Whether it’s studying the behavior of substances at different temperatures, designing new materials, or cooking, knowing how matter changes from one state to another is essential.
Physical and Chemical Changes

When discussing matter and change, it is important to differentiate between physical and chemical changes. Physical changes are those that do not result in the formation of new substances. The composition of the matter remains the same, although its physical properties may be altered. Examples of physical changes include changes in state (such as melting or freezing), changes in shape or size (such as cutting or crushing), and changes in appearance (such as dissolving or distilling).
On the other hand, chemical changes involve the formation of new substances with different chemical properties. During a chemical change, the composition of the matter is altered, and new substances are formed. This can be observed through changes in color, the production of gas or odor, the release or absorption of energy, or the formation of a precipitate. Examples of chemical changes include rusting of iron, burning of wood, and digestion of food.
In summary, physical changes involve alterations in the physical properties of matter without the formation of new substances, while chemical changes involve the formation of new substances with different chemical properties. Both types of changes are important in understanding the behavior and transformations of matter.
Conservation of Mass

The conservation of mass, also known as the law of mass conservation, is a fundamental concept in chemistry. It states that the total mass of a closed system remains constant during a chemical reaction or physical change. In other words, matter cannot be created or destroyed, only transformed from one form to another.
This principle is based on the idea that atoms are the building blocks of matter and cannot be created or destroyed. During a chemical reaction, the rearrangement of these atoms may result in the formation or dissolution of compounds, but the total number of atoms remains the same. This is why chemical equations must be balanced – to ensure that the number of atoms on both sides of the equation is equal.
For example, in the combustion of methane gas (CH4), the reactant methane combines with oxygen gas (O2) to produce carbon dioxide (CO2) and water (H2O). In this reaction, the total number of carbon, hydrogen, and oxygen atoms remains constant, even though the arrangement of these atoms changes. This exemplifies the conservation of mass.
The conservation of mass is a fundamental principle that is used to study and understand various chemical phenomena. It allows scientists to predict and calculate the quantities of reactants and products involved in a chemical reaction. Additionally, it serves as the basis for stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction.
Solutions and Mixtures
In this chapter, we have explored the concepts of solutions and mixtures. A solution is a homogeneous mixture composed of two or more substances. A mixture, on the other hand, can be homogeneous or heterogeneous and is composed of two or more substances that are not chemically combined.
We have learned about the different types of mixtures, including suspensions, colloids, and solutions. Suspensions are heterogeneous mixtures that contain larger particles that can be seen easily and will eventually settle. Colloids are heterogeneous mixtures that contain smaller particles that do not settle and can scatter light. Solutions, on the other hand, are homogeneous mixtures that contain smaller particles that do not settle and are not visible.
Throughout this chapter, we have explored various properties of solutions, such as solubility, concentration, and saturation. We have also discussed the factors that affect solubility, such as temperature and pressure.
In conclusion, solutions and mixtures play a crucial role in our everyday lives. From the air we breathe to the drinks we consume, solutions and mixtures surround us. Understanding the properties and behavior of solutions and mixtures can help us in various fields, including chemistry, medicine, and environmental science.