In Chapter 14, we delve into the fascinating world of acids and bases. Acids and bases play a fundamental role in chemistry, shaping the way we understand chemical reactions and their effects. This chapter provides an answer key to help guide you through the challenging concepts and problems that arise when studying acids and bases.
Acids and bases are everywhere in our daily lives – from the food we eat to the products we use. Understanding their properties and reactions is essential for a deeper understanding of chemistry. This answer key will help you master the principles of acids and bases, including their definitions, calculations, and common laboratory techniques.
One of the key focuses of this chapter is understanding the pH scale – a measure of the acidity or alkalinity of a substance. By providing step-by-step solutions to the exercises and problems related to the pH scale, this answer key will give you the confidence to navigate the complex world of acids and bases.
Chapter 14 Acids and Bases Answer Key
In Chapter 14, we explore the fundamentals of acids and bases, their properties, and their behavior in various chemical reactions. Acids and bases play a crucial role in chemistry and are widely used in everyday life. This answer key serves as a guide to understanding the concepts and solving problems related to this chapter.
Section 14.1: Definitions of Acids and Bases
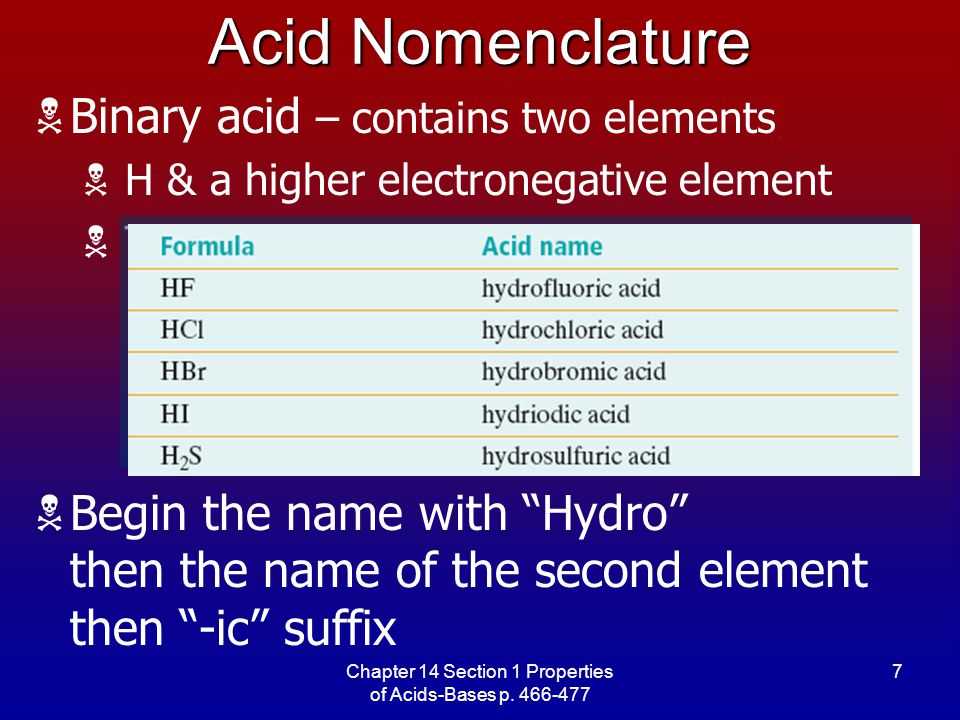
- An acid is a compound that donates a proton (H+) in a chemical reaction.
- A base is a compound that accepts a proton (H+) in a chemical reaction.
- The Arrhenius definition defines acids as compounds that produce H+ ions in aqueous solutions, and bases as compounds that produce OH- ions in aqueous solutions.
- The Brønsted-Lowry definition defines acids as proton donors and bases as proton acceptors.
Section 14.2: Properties of Acids and Bases
- Acids have a sour taste and turn blue litmus paper red.
- Bases have a bitter taste and turn red litmus paper blue.
- Acids react with metals to produce hydrogen gas.
- Bases are slippery to the touch.
- Acids and bases can undergo neutralization reactions to form salts and water.
Section 14.3: Acid-Base Reactions and pH
- An acid-base reaction involves the transfer of a proton from an acid to a base.
- The pH scale measures the acidity or basicity of a solution.
- A pH less than 7 indicates acidity, a pH greater than 7 indicates basicity, and a pH of 7 indicates neutrality.
- pH can be calculated using the equation pH = -log[H+].
Section 14.4: Acid-Base Titrations
- An acid-base titration is a technique used to determine the concentration of an acid or base in a solution.
- A titration involves the addition of a known volume of a solution with a known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction is complete.
- The equivalence point is reached when the moles of acid are equal to the moles of base or vice versa.
- Indicators, such as phenolphthalein or bromothymol blue, are used to visually indicate the completion of the reaction.
Section 14.5: Buffers
- A buffer is a solution that resists changes in pH when small amounts of acid or base are added.
- Buffers are usually a combination of a weak acid and its conjugate base or a weak base and its conjugate acid.
- Buffer capacity refers to the ability of a buffer solution to resist changes in pH.
This answer key provides a comprehensive overview of the key concepts and principles covered in Chapter 14 of acids and bases. By understanding these concepts and practicing problem-solving techniques, you will be well-prepared to tackle any acid-base-related challenges.
Overview
Chapter 14 of the textbook covers the topic of acids and bases. Acids and bases are essential components of chemistry and have numerous applications in various fields. This chapter provides a comprehensive understanding of the properties, behavior, and reactions of acids and bases.
The chapter begins with an introduction to acids and bases, explaining their definitions and characteristics. It explores the different theories of acids and bases, including the Arrhenius, Bronsted-Lowry, and Lewis theories. These theories lay the foundation for understanding acid-base reactions.
The chapter then delves into the pH scale and how it is used to measure the acidity or basicity of a solution. It discusses the calculations involved in determining pH and pOH values and explores the relationship between pH and hydrogen ion concentration. Additionally, it covers the concept of neutralization reactions and how they occur between acids and bases.
The chapter also covers various acid-base equilibrium concepts, such as the equilibrium constant and the ionization of weak acids and bases. It explores the factors that influence the strength of acids and bases, including their molecular structure and concentration. The concept of acid-base titrations is introduced, along with the calculations involved in determining the concentration of an unknown solution.
Overall, Chapter 14 provides a comprehensive overview of acids and bases, exploring their properties, reactions, and applications. It equips students with the knowledge and understanding to analyze and predict the behavior of acids and bases in different scenarios, ensuring a solid foundation in this fundamental area of chemistry.
Acids and Bases: Definitions and Properties
Acids and bases are two types of compounds that play important roles in chemistry and everyday life. They can be defined and characterized based on their properties and behavior in various chemical reactions.
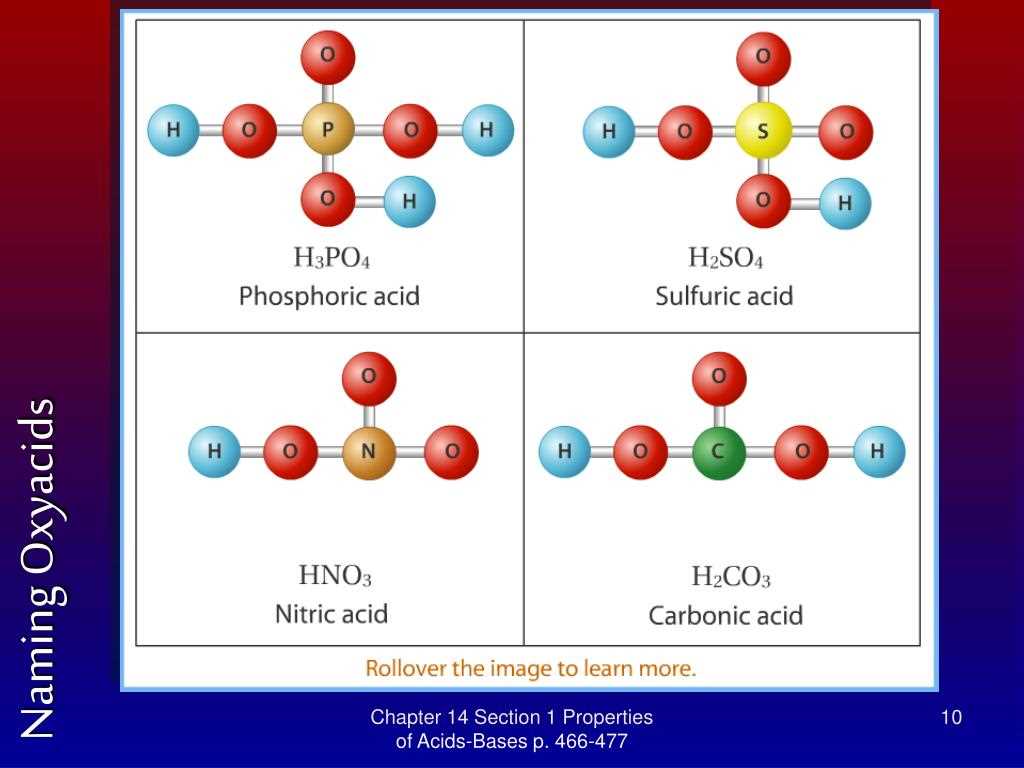
An acid is a substance that donates hydrogen ions (H+) in a chemical reaction. This can be seen in the equation: acid + water → H+ + anion. Acids have certain properties that distinguish them from other compounds. They taste sour, turn blue litmus paper red, and react with bases to form salt and water. Common examples of acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
A base, on the other hand, is a substance that accepts hydrogen ions (H+) in a chemical reaction. The equation for the reaction between a base and water is: base + water → hydroxide ion (OH-) + cation. Bases have their own set of properties. They taste bitter, feel slippery, and turn red litmus paper blue. Common examples of bases include sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2).
Both acids and bases can be classified as strong or weak based on their ability to donate or accept hydrogen ions. Strong acids and bases completely dissociate in water, while weak acids and bases only partially dissociate. pH is a measure of the concentration of hydrogen ions in a solution and ranges from 0 to 14. Acids have a pH less than 7, bases have a pH greater than 7, and a pH of 7 is considered neutral.
Understanding the definitions and properties of acids and bases is essential in many areas of science, from chemistry to biology. It allows us to explain and predict the behavior of substances in various chemical reactions and understand their impact on our daily lives.
Strength of Acids and Bases
The strength of acids and bases refers to their ability to dissociate in water and release hydrogen ions (H+) or hydroxide ions (OH-). Strong acids and bases completely dissociate in water, while weak acids and bases only partially dissociate.
Acidity is measured on a pH scale, which ranges from 0 to 14. Acids with a pH value closer to 0 are stronger, while those closer to 7 are weaker. Similarly, bases with a pH closer to 14 are stronger, while those closer to 7 are weaker. The strength of an acid or base can be determined by its ability to donate or accept protons.
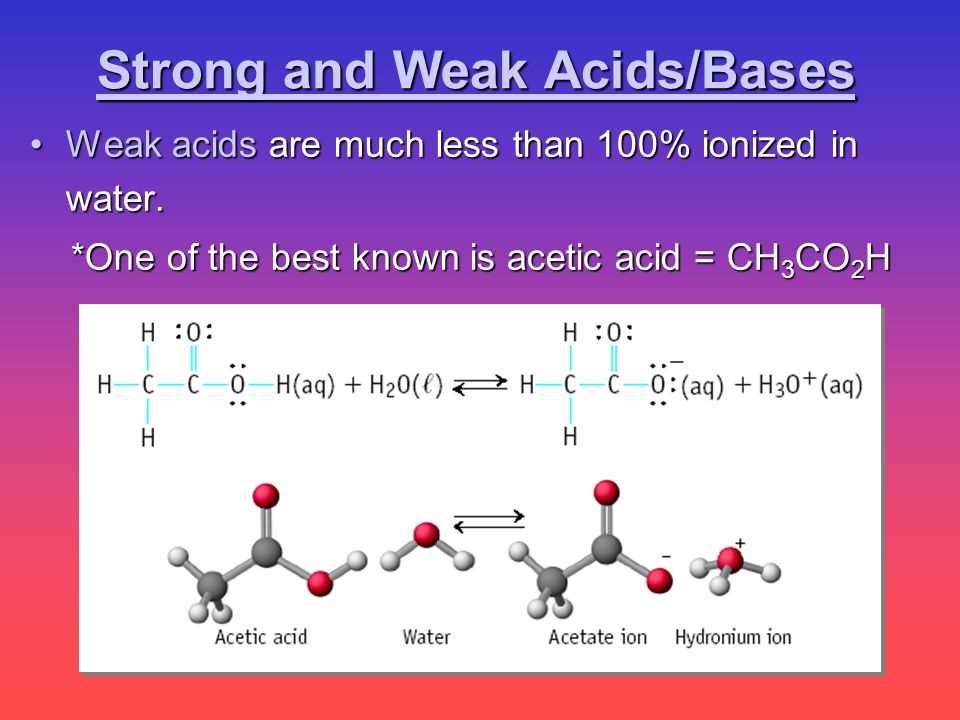
Some common examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). These acids ionize completely when dissolved in water, releasing a high concentration of H+ ions. In contrast, weak acids like acetic acid (CH3COOH) only partially ionize, resulting in a lower concentration of H+ ions.
On the other hand, strong bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2). These bases dissociate completely in water, producing a high concentration of OH- ions. Weak bases such as ammonia (NH3) only partially dissociate, leading to a lower concentration of OH- ions.
In summary, the strength of acids and bases is determined by their ability to ionize in water. Strong acids and bases fully dissociate, while weak acids and bases only partially dissociate. The pH scale is used to measure acidity, with lower values indicating stronger acids and higher values indicating stronger bases.
Acid-Base Reactions
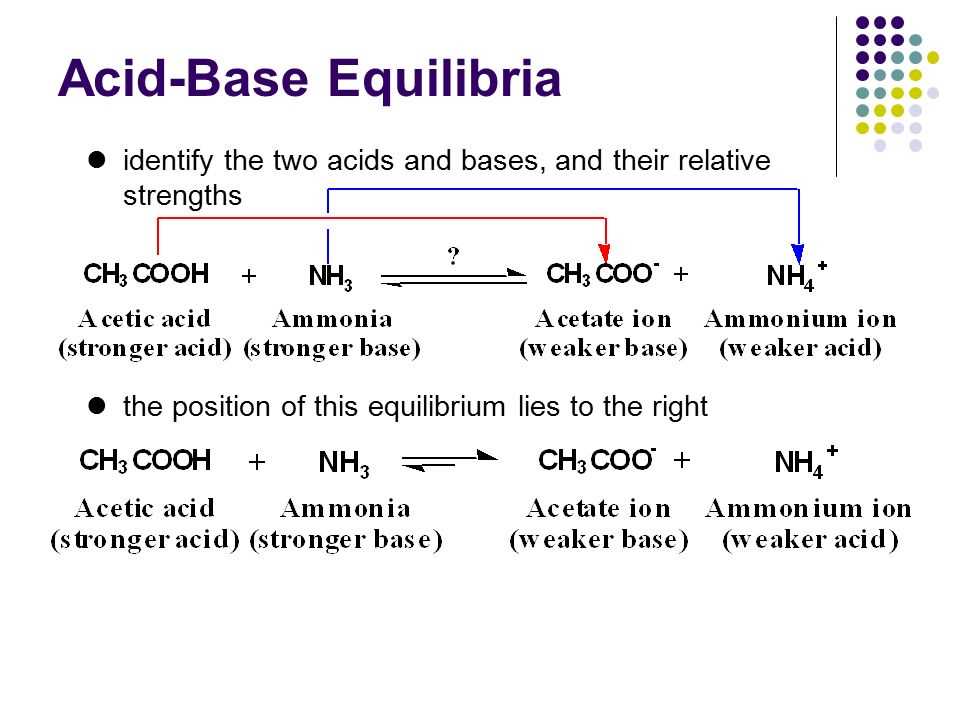
In chemistry, acid-base reactions are an essential part of understanding the behavior of chemicals. These reactions involve the transfer of protons (H+) from an acid to a base, resulting in the formation of a new compound. The key to understanding acid-base reactions is recognizing the presence of acids and bases, as well as their respective strengths.
An acid is defined as a substance that donates protons, while a base is a substance that accepts protons. Acids and bases can be further classified as strong or weak, based on their ability to dissociate in water. Strong acids completely dissociate into ions when dissolved in water, while weak acids only partially dissociate. Similarly, strong bases completely dissociate into hydroxide ions (OH-) when dissolved in water, while weak bases only partially dissociate.
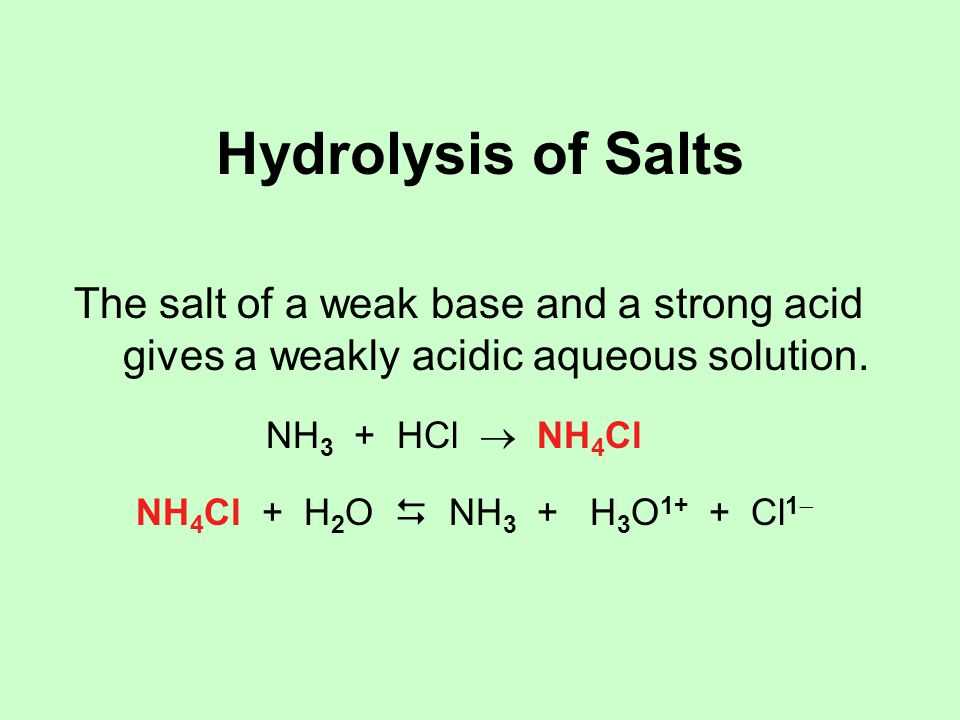
The reaction between an acid and a base is commonly referred to as a neutralization reaction. In this type of reaction, the H+ ions from the acid combine with the OH- ions from the base to form water (H2O). The remaining ions from the acid and base combine to form a salt. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are water and sodium chloride (NaCl).
Acid-base reactions are not only important in chemistry but also play a vital role in our everyday lives. For example, the process of digestion in our stomach involves the production of hydrochloric acid, which helps break down food. Additionally, many household cleaning products are either acids or bases, which interact with dirt and stains.
In conclusion, acid-base reactions are fundamental in chemistry, involving the transfer of protons from acids to bases. The classification of acids and bases as strong or weak is crucial for understanding the extent of dissociation. Neutralization reactions result in the formation of water and a salt and have various applications in biology, chemistry, and everyday life.
Calculations Involving Acids and Bases
When working with acids and bases, it is important to be able to perform calculations to determine various properties and quantities. These calculations are based on the principles of acid-base reactions and the concept of concentration.
One common type of calculation involves determining the pH of an acidic or basic solution. pH is a measure of the acidity or alkalinity of a solution and is determined using the logarithmic scale. The pH scale ranges from 0 to 14, with 0 being the most acidic, 14 being the most alkaline, and 7 being neutral. To calculate pH, you can use the equation pH = -log[H+], where [H+] represents the concentration of hydrogen ions in the solution.
In addition to pH calculations, it is also important to be able to determine the concentration of acids and bases. The concentration of an acid or base can be expressed in terms of molarity (M), which is defined as the number of moles of solute per liter of solution. To calculate the molarity of an acid or base, you can use the equation M = n/V, where n represents the number of moles of solute and V represents the volume of the solution in liters.
Another important calculation involving acids and bases is the stoichiometry of acid-base reactions. This involves determining the ratios of reactants and products in a chemical reaction. To perform these calculations, you need to use balanced chemical equations and mole ratios. By performing stoichiometric calculations, you can determine the amount of acid or base needed to react with a given amount of another substance, or the amount of acid or base produced in a reaction.
In conclusion, calculations involving acids and bases are essential for understanding and determining various properties and quantities. These calculations include determining the pH of a solution, calculating the concentration of acids and bases, and performing stoichiometric calculations. By mastering these calculations, you can gain a deeper understanding of the behavior and characteristics of acids and bases.
The Acid-Base Balance: Buffers
In Chapter 14, we have explored the concept of acids and bases and their properties. We have learned that acids are substances that can donate protons (H+) and bases are substances that can accept protons. Acids and bases play a crucial role in maintaining the pH balance in various biological systems.
One important aspect of the acid-base balance is the concept of buffers. Buffers are solutions that are able to resist changes in pH when an acid or base is added. They help maintain the pH of a solution within a narrow range, which is important for the proper functioning of biological systems.
Buffers work by having a combination of a weak acid and its conjugate base or a weak base and its conjugate acid. When an acid is added to a buffer solution, the weak base component of the buffer reacts with the added acid, preventing a significant decrease in pH. Similarly, when a base is added, the weak acid component of the buffer reacts with the added base, preventing a significant increase in pH.
Buffers are crucial in maintaining the acid-base balance in various biological processes. For example, in the human body, blood contains several buffer systems that help regulate the pH of the blood. These buffers, such as the bicarbonate buffer system, help maintain the blood pH between a narrow range of 7.35-7.45.
Overall, buffers are essential for the proper functioning of biological systems by ensuring the maintenance of a stable pH. They play a vital role in preventing significant changes in pH, which can be harmful to cells and biological processes. Understanding buffers and their role in the acid-base balance is important in various fields such as medicine, biochemistry, and environmental science.
Q&A:
What is a buffer?
A buffer is a substance that helps to maintain the pH balance of a solution by resisting changes in its acidity or alkalinity.
How do buffers work?
Buffers work by either accepting or donating protons (H+ ions), thereby stabilizing the pH of a solution.
What is the importance of buffers in the body?
Buffers are crucial for maintaining the acid-base balance in the body, as they help to regulate the pH of bodily fluids such as blood and urine.
What are common examples of buffers?
Common examples of buffers include bicarbonate ions in the blood, which help regulate the pH, and phosphate buffer systems found in cells.
What is the role of buffers in the digestive system?
Buffers in the digestive system help to maintain the pH balance in the stomach and small intestine, allowing for optimal enzyme activity and digestion.
What are buffers?
Buffers are substances or systems that help maintain a stable pH in a solution by resisting changes in acidity or alkalinity.
What is the acid-base balance?
The acid-base balance refers to the balance between acids and bases in the body, which is crucial for maintaining proper physiological function. It involves the regulation of hydrogen ion concentration (pH) in bodily fluids.