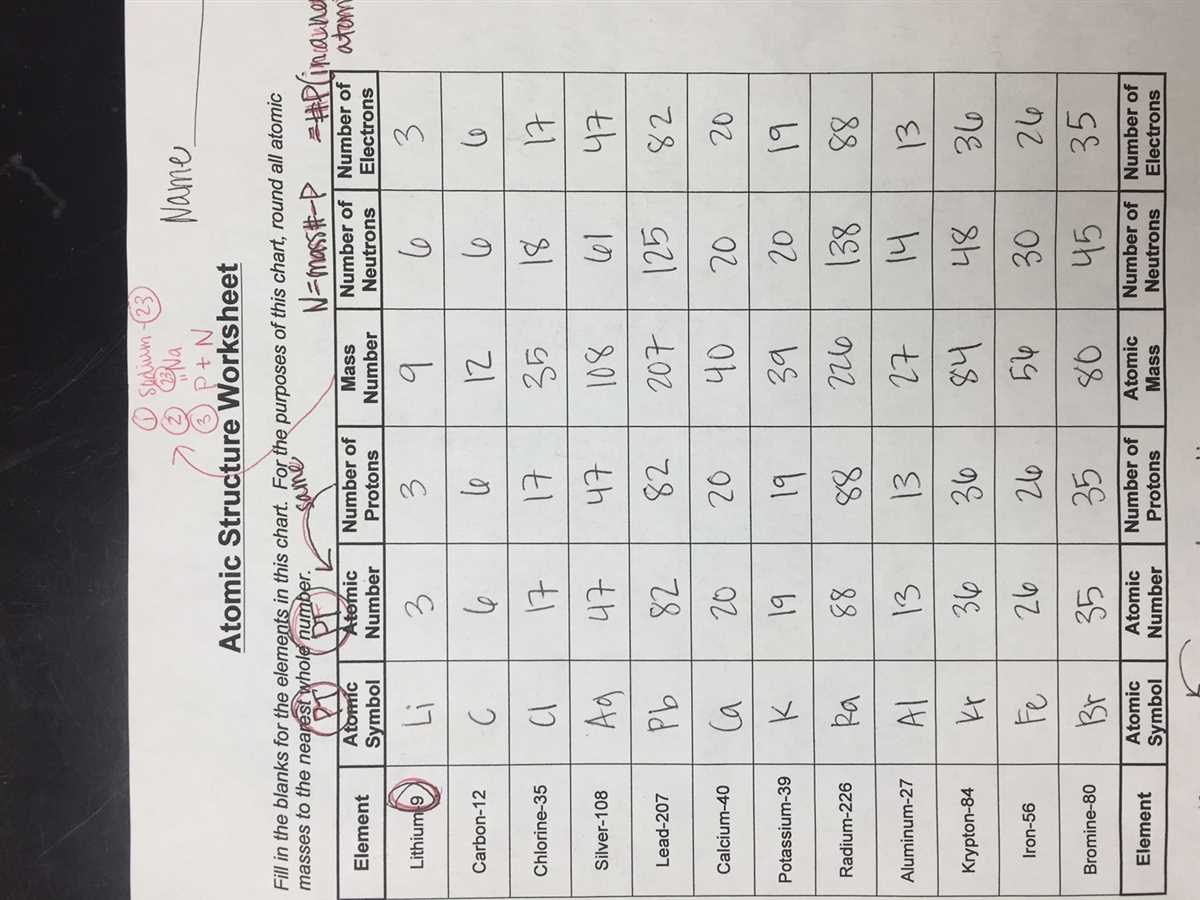
Welcome to our atoms and ions worksheet answer key! In this article, we will be providing you with the answers to a worksheet that focuses on atoms and ions. This worksheet is designed to test your knowledge and understanding of the fundamental concepts of atoms and ions, including their properties, charges, and formation.
Atoms are the smallest units of matter that retain the properties and characteristics of an element. They are made up of three subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its atomic number and identifies the element.
Ions, on the other hand, are atoms that have gained or lost electrons, resulting in a net positive or negative charge. When an atom loses electrons, it becomes a cation and has a positive charge. Conversely, when an atom gains electrons, it becomes an anion and has a negative charge. The charges of ions are indicated by superscripts and are shown after the atomic symbol in a chemical formula.
In the following section, we will provide you with the answers to the atoms and ions worksheet. It is important to remember that these answers are only a guide and may vary depending on your specific worksheet or textbook. Let’s dive in!
Understanding Atoms and Ions: A Worksheet Answer Key
In the study of chemistry, atoms and ions are fundamental concepts that help us understand the behavior of matter at a microscopic level. Atoms are the building blocks of matter, while ions are charged atoms or molecules that have gained or lost electrons. In this worksheet answer key, we will delve deeper into the concepts of atoms and ions and explore their properties and behavior.
1. Atoms:
Atoms are the smallest unit of an element that retains its chemical properties. They consist of three fundamental subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its atomic number, which determines the element’s identity. The total number of protons and neutrons in an atom is called its atomic mass.
2. Ions:
Ions are formed when atoms gain or lose electrons. If an atom gains electrons, it becomes a negatively charged ion called an anion. If an atom loses electrons, it becomes a positively charged ion called a cation. The charge of an ion depends on the number of electrons gained or lost. Ions play a crucial role in chemical reactions and bonding, as they can attract or repel other ions based on their charges.
3. Ionization and Ionic Compounds:
The process of gaining or losing electrons to form ions is called ionization. When ions of opposite charges attract each other, they form ionic compounds through electrostatic forces. These compounds have a regular lattice structure composed of alternating positive and negative ions. Examples of ionic compounds include table salt (sodium chloride) and calcium carbonate (found in limestone).
4. Ion Charges and Periodic Table:
The charges of ions can be determined based on their positions in the periodic table. Some elements have fixed charges, such as the alkali metals, which always form +1 ions. Transition metals, on the other hand, can have multiple charges and form ions with different charges. The periodic table provides valuable information about the properties and behaviors of different elements and their ions.
By understanding the concepts of atoms and ions, we can gain a deeper understanding of the behavior of matter and the chemical reactions that occur in our everyday lives. This worksheet answer key serves as a guide to help students grasp these concepts and apply them to various problem-solving scenarios in chemistry.
Exploring Atomic Structure
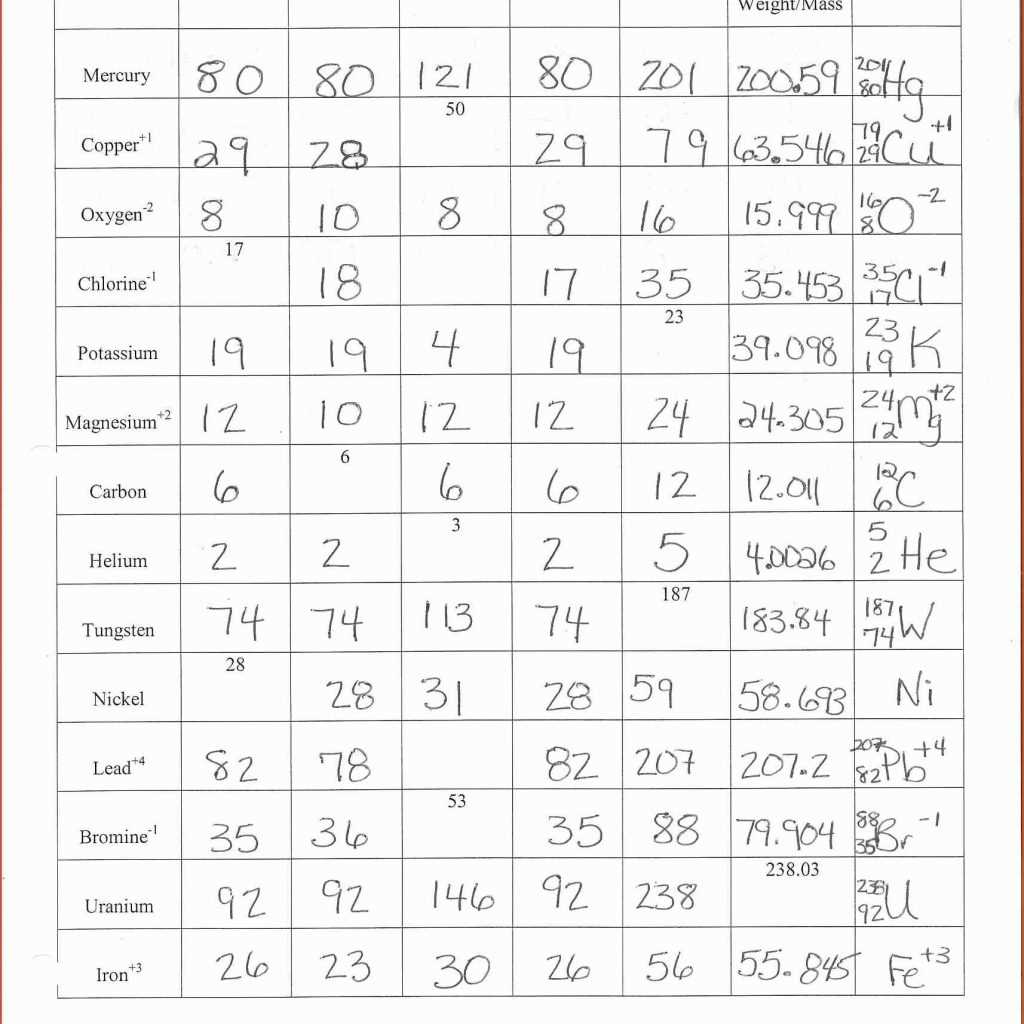
The study of atomic structure is essential to understanding the behavior and properties of matter. Atoms are the basic building blocks of all matter, and they consist of three subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
One way to explore atomic structure is through the use of the periodic table. The periodic table organizes all known elements based on their atomic number, which is the number of protons in an atom. The number of protons determines the identity of an element. For example, hydrogen has one proton, carbon has six protons, and gold has 79 protons.
Another important concept in atomic structure is electron configuration. Electrons are arranged in energy levels or shells around the nucleus of an atom. The first energy level can hold a maximum of 2 electrons, the second energy level can hold a maximum of 8 electrons, and the third energy level can hold a maximum of 18 electrons. Electrons fill the energy levels in a specific order, starting with the lowest energy level.
In conclusion, exploring atomic structure is crucial for understanding the fundamental properties of matter. The study of protons, neutrons, and electrons, as well as the organization of elements in the periodic table and electron configuration, provides a foundation for further research and discovery in the field of chemistry.
Types of Ions
In the study of atoms and molecules, ions play a crucial role. An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net positive or negative charge. There are two main types of ions: cations and anions.
Cations: Cations are ions that have a net positive charge. This means they have lost one or more electrons and have more protons than electrons. Cations are typically formed when a metal atom loses one or more electrons. For example, when a sodium atom (11 protons, 11 electrons) loses one electron, it becomes a sodium cation with a net charge of +1. Cations are attracted to negatively charged particles and are often involved in chemical reactions.
Anions: Anions are ions that have a net negative charge. This means they have gained one or more electrons and have more electrons than protons. Anions are typically formed when a nonmetal atom gains one or more electrons. For example, when a chlorine atom (17 protons, 17 electrons) gains one electron, it becomes a chloride anion with a net charge of -1. Anions are attracted to positively charged particles and can also be involved in chemical reactions.
It is important to note that the charge of an ion is indicated by a superscript next to its symbol. For example, the sodium ion mentioned earlier would be represented as Na+ and the chloride ion would be represented as Cl–.
In conclusion, cations and anions are the two main types of ions. Cations have a net positive charge and are formed when a metal atom loses electrons, while anions have a net negative charge and are formed when a nonmetal atom gains electrons. These ions play key roles in various chemical reactions and interactions.
Balancing Ionic Equations
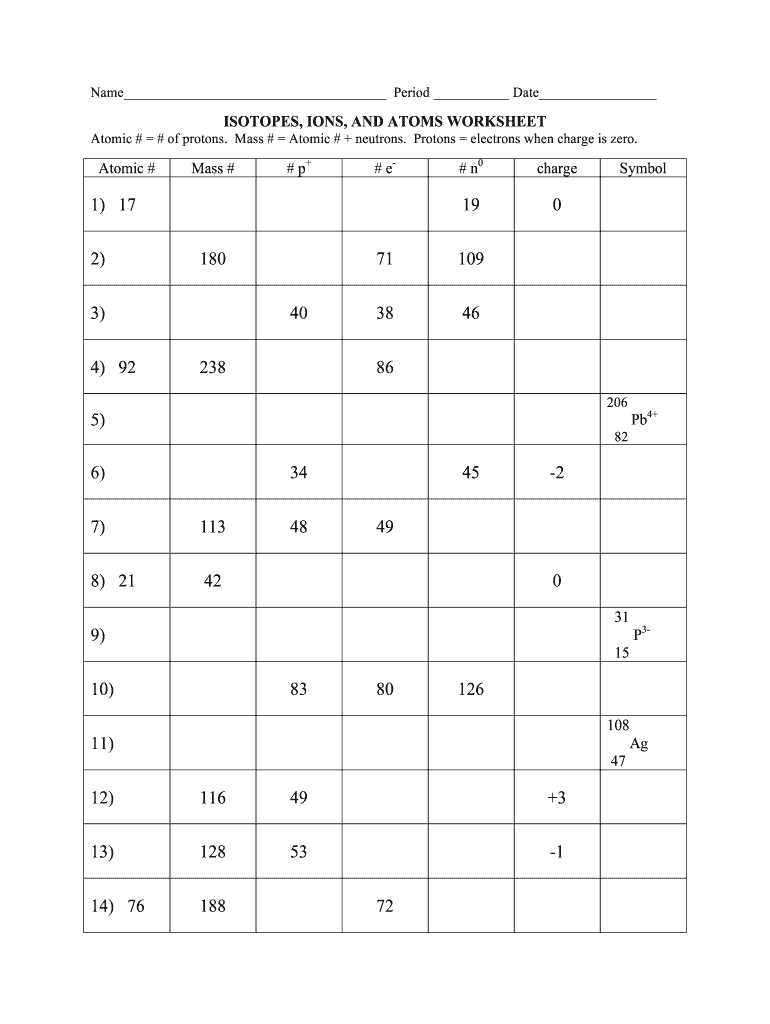
When working with ionic compounds, it is important to balance the equations to accurately represent the chemical reactions that take place. Balancing ionic equations involves ensuring that the number of atoms and charges on both sides of the equation are equal.
One of the first steps in balancing an ionic equation is to identify the ions present in the reaction. This can be done by breaking down the ionic compounds into their individual ions. For example, in the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3), the ions involved are sodium (Na+), chloride (Cl-), silver (Ag+), and nitrate (NO3-).
Once the ions are identified, it is necessary to balance the charges on both sides of the equation. This is achieved by adjusting the coefficients in front of the ions or compounds. For example, if there are two nitrate ions on the left side of the equation, there should also be two nitrate ions on the right side to balance the charges.
In some cases, it may be necessary to introduce additional compounds or ions to balance the equation. These additional compounds are called spectator ions and do not participate in the chemical reaction. They are present to balance the charges and ensure that the number of atoms and charges are equal on both sides of the equation.
Balancing ionic equations requires careful attention to detail and an understanding of the charges and properties of the ions involved. It is an important skill in chemistry and is necessary for accurately representing chemical reactions.
Conducting Ion Experiments

Ions are electrically charged particles that are formed when atoms gain or lose electrons. Conducting ion experiments is an important way to study and understand the behavior of ions in various chemical reactions. These experiments allow scientists to manipulate ions and observe their interactions with other substances.
One type of ion experiment involves studying the conductivity of different solutions. In this experiment, various substances are dissolved in water to create different solutions. An electrical current is then passed through the solutions, and the conductivity is measured. This allows scientists to determine if the substances in the solutions are ions or not. The conductivity of a solution depends on the presence and concentration of ions in the solution.
Another type of ion experiment focuses on the reactivity of ions. In this experiment, different ions are mixed together and their reactions are observed. For example, by mixing a solution of sodium chloride (NaCl) with a solution of silver nitrate (AgNO3), a reaction occurs where silver chloride (AgCl) precipitates out of the solution. This experiment helps chemists understand the ability of ions to form new compounds and the specific reactions that occur.
Ion experiments are not only conducted in a laboratory setting. They can also be done at home or in a classroom using simple materials. For example, students can use a battery, wires, and graphite electrodes to create a simple circuit and test the conductivity of different solutions. They can also mix solutions of various ions and observe any visible reactions.
By conducting ion experiments, scientists and students are able to deepen their understanding of the behavior of ions and their role in chemical reactions. These experiments provide valuable insights into the properties and reactivity of ions, helping to expand our knowledge of the building blocks of matter.
Applying Knowledge to Real-World Scenarios

Understanding atoms and ions is not just a theoretical concept. It has numerous real-world applications in various fields. Let’s explore a few scenarios where this knowledge plays a crucial role:
- Chemistry and Chemical Reactions: Understanding atoms and ions is essential in the field of chemistry. It allows scientists to predict and explain chemical reactions, understand the behavior of compounds, and design new materials. Without this knowledge, advancements in fields like medicine, industry, and energy would not be possible.
- Environmental Science: Atoms and ions are involved in various environmental processes. Studying their behavior helps us understand and find solutions to issues like pollution, acid rain, and climate change. For example, knowledge of ion exchange processes in soil can help in designing effective wastewater treatment plants.
- Electronics and Nanotechnology: In the field of electronics, atoms and ions are the building blocks of transistors, computer chips, and other electronic devices. Understanding their behavior and manipulation is crucial for developing smaller and more efficient technology. Moreover, nanotechnology relies heavily on manipulating atoms and ions to create new materials and devices at the nanoscale.
- Medicine and Pharmacology: Understanding atoms and ions is essential in the field of medicine. It allows scientists to design drugs that target specific ions or atoms in the body, thus treating diseases more effectively. Additionally, knowledge of ion channels in cells helps in understanding how drugs interact with cellular processes.
In conclusion, atoms and ions have a significant impact on our lives and various fields of science. Their understanding enables advancements in technology, medicine, and environmental sciences. By studying their behavior and applying this knowledge, we can tackle real-world challenges and make important discoveries.