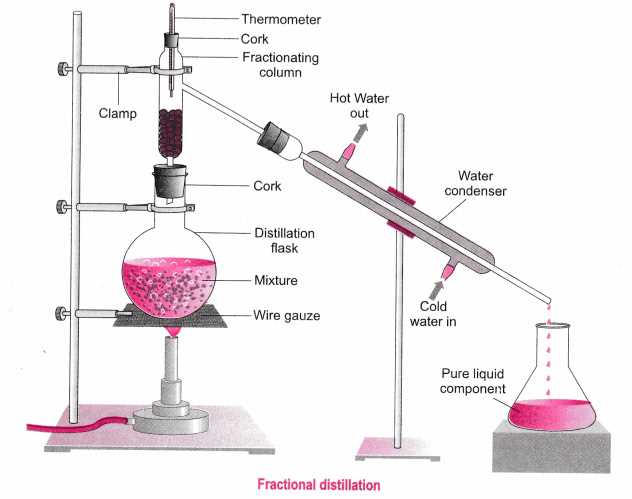
Lab 2 in Chemistry 1 focuses on the separation of mixtures to further understand the different components that make up a mixture. This lab helps students gain a hands-on experience in separating mixtures using various separation techniques.
The objective of Lab 2 is to separate a mixture into its individual components through methods such as filtration, evaporation, centrifugation, and chromatography. By performing these techniques, students can identify the specific properties of each component and understand how they can be isolated from the mixture.
Throughout the lab, students will follow a series of steps and record their observations. These observations will then be used to analyze and explain the separation process. Additionally, students will answer questions related to the separation techniques used and the properties of the components in the mixture.
By completing Lab 2, students will gain a deeper understanding of the principles of separation and have the opportunity to apply their knowledge in a practical setting. This lab is crucial in developing fundamental skills in chemistry and lays the foundation for more advanced experiments in the future.
Lab 2 Separation of a Mixture Chemistry 1 Answers

The laboratory 2 experiment focused on the separation of a mixture in Chemistry 1. The objective was to apply various separation techniques to separate a mixture of substances into its individual components.
In this lab, we were provided with a mixture of sand, salt, and iron filings. The first step was to use a magnet to separate the iron filings from the mixture. We passed a magnet over the mixture, and the iron filings were attracted to the magnet, allowing us to easily remove them.
After separating the iron filings, we focused on separating the remaining sand and salt. We dissolved the mixture in water to separate the salt, as salt is soluble in water. We then used a filtration technique to remove the sand from the mixture. By pouring the mixture through a filter paper, the sand was retained on the filter while the saltwater solution passed through.
Lastly, we needed to separate the salt from the water. We evaporated the water by heating the saltwater solution, allowing the water to evaporate and leaving behind the salt crystals. The salt crystals were then collected and weighed, providing us with the final component of the mixture.
In conclusion, the lab 2 experiment demonstrated various separation techniques that can be applied to separate a mixture of substances. By using a magnet, filtration, and evaporation, we were able to successfully separate the iron filings, sand, and salt from the initial mixture.
What is Lab 2: Separation of a Mixture?
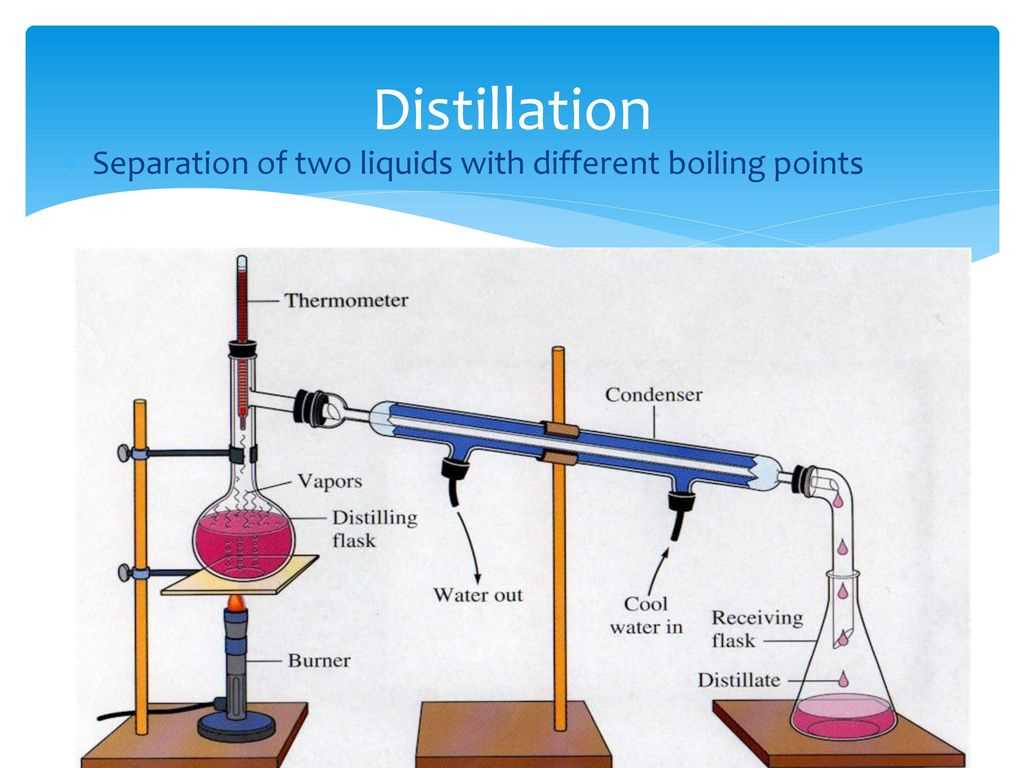
Lab 2: Separation of a Mixture is a laboratory experiment in the field of chemistry that focuses on the process of separating a mixture into its individual components. In this lab, students are given a mixture of two or more substances and are tasked with using various separation techniques to isolate and identify the different components.
The goal of this lab is to teach students about the different methods of separation that can be used to isolate the substances in a mixture. This includes techniques such as filtration, distillation, chromatography, and evaporation. By performing these techniques and analyzing the results, students gain a better understanding of the physical and chemical properties of the substances present in the mixture.
During the lab, students carefully follow a set of instructions and perform the necessary procedures to separate the mixture. This may involve heating, cooling, filtering, or using different solvents to dissolve specific components. Throughout the process, students also make observations and record data, which they later analyze and interpret to draw conclusions about the nature of the mixture.
Key objectives of Lab 2: Separation of a Mixture may include:
- Understanding the concept of mixtures and their components
- Learning various separation techniques and when to use them
- Developing skills in following experimental procedures and making accurate observations
- Analyzing data and drawing conclusions based on the results
- Enhancing problem-solving and critical thinking skills
In conclusion, Lab 2: Separation of a Mixture is an important experiment in chemistry education that teaches students essential skills and knowledge related to the separation of mixtures. By performing the necessary techniques and analyzing the results, students gain a deeper understanding of the properties of different substances and the methods used to separate them. This lab serves as a foundation for further exploration and study in the field of chemistry.
Importance of Lab 2: Separation of a Mixture
The Lab 2: Separation of a Mixture is an essential experiment in the field of chemistry. It allows students to gain hands-on experience in separating different components of a mixture using various techniques. This lab is crucial in developing fundamental skills and understanding of the principles of separation and purification processes in chemistry.
One of the primary reasons why this lab is important is that it teaches students how to separate mixtures into their individual components. Mixtures are often found in nature and in various industrial processes. By knowing how to separate the components of a mixture, chemists can identify and isolate specific substances for further analysis or use. This knowledge is vital in fields like pharmaceuticals, environmental science, and forensic chemistry.
The lab also introduces students to different separation techniques such as filtration, evaporation, and chromatography. These techniques are used extensively in research and industrial settings to isolate and purify substances. By performing these techniques in the lab, students gain practical skills that can be applied in real-world scenarios.
Furthermore, Lab 2: Separation of a Mixture helps students develop critical thinking and problem-solving skills. They need to analyze the properties of the mixture and select the most appropriate separation technique for each component. This process requires careful observation, data analysis, and decision-making. These skills are essential for success in scientific research and other analytical professions.
In conclusion, Lab 2: Separation of a Mixture is important because it provides students with practical knowledge and skills in separation techniques, helps in understanding the principles of separation and purification, and enhances critical thinking abilities. This lab sets a solid foundation for further studies in chemistry and prepares students for future scientific endeavors.
Methodology of Lab 2: Separation of a Mixture
In Lab 2, we conducted an experiment to separate a mixture of solids. The purpose of this experiment was to learn and apply different separation techniques to isolate the individual components of the mixture.
We started by carefully observing the mixture and recording its physical properties, such as color, texture, and appearance. This initial step was important in order to have a better understanding of the mixture and to determine which separation methods would be most appropriate.
To separate the mixture, we utilized various techniques, including filtration, evaporation, and magnetism. Filtration was used to separate insoluble solids from a liquid. We poured the mixture through a filter paper, which allowed the liquid to pass through while retaining the solid particles. This technique was suitable for mixtures where the solid was much larger than the liquid particles.
Evaporation was employed to separate a soluble solid from a liquid. We heated the mixture in a beaker, allowing the liquid to evaporate and leaving behind the solid residue. This method was particularly effective when the desired solid had a significantly higher boiling point than the liquid.
Magnetism was utilized when one or more components of the mixture had magnetic properties. By using a magnet, we were able to attract and separate the magnetic components from the rest of the mixture.
Overall, through the use of these separation techniques, we were able to successfully isolate the individual components of the mixture. This experiment provided us with valuable hands-on experience in the field of chemistry and helped reinforce the importance of proper observation and utilization of appropriate separation methods.
Result and Analysis of Lab 2: Separation of a Mixture
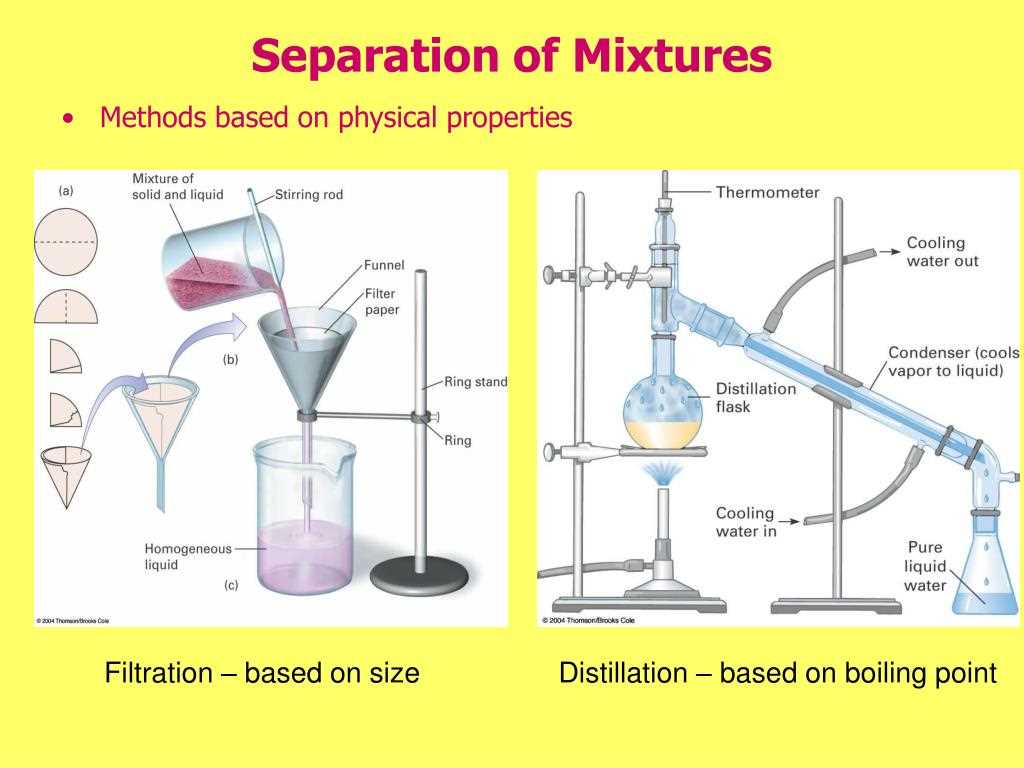
In Lab 2, the goal was to separate a mixture of sand, salt, and iron filings using various physical separation techniques. The mixture was first homogenized and then separated through processes such as filtration, evaporation, and magnetic separation.
The first step in the separation process was to use a magnet to attract and collect the iron filings. This was successful, as the iron filings were easily separated from the rest of the mixture. This method is based on the property of magnetism, where iron filings are attracted to a magnet.
After removing the iron filings, the remaining mixture was then poured into a funnel with filter paper to perform filtration. Filtration was used to separate the sand from the salt solution. The sand particles were too large to pass through the small pores of the filter paper, so they were retained on the filter while the salt solution passed through. This technique relies on the difference in particle size between the sand and the salt.
The final step of separation involved evaporating the salt solution to retrieve the salt. The mixture was heated in a beaker, and as the solution evaporated, salt crystals formed and were left behind in the container. This process takes advantage of the different boiling points of water and salt, allowing for the separation of the two substances.
In conclusion, the lab experiment successfully separated the sand, salt, and iron filings in the mixture using techniques such as magnetic separation, filtration, and evaporation. These physical separation methods were effective in isolating each component based on their unique properties. The experiment highlights the importance of understanding the properties of different substances in order to separate mixtures efficiently.
Discussion on Lab 2: Separation of a Mixture
In Lab 2, we explored the concept of separating a mixture through various physical separation techniques. The main objective of this lab was to separate a mixture of salt, sand, and iron filings using methods such as filtration, magnetic separation, and evaporation.
We began the experiment by creating the mixture and then carrying out each separation technique one by one. Filtration was used to separate the sand from the mixture, as the sand particles were larger and could not pass through the small pores of the filter paper. This technique allowed us to obtain a filtrate containing the salt and water mixture, which was then subjected to evaporation. The water evaporated, leaving behind the salt crystals that could be collected and weighed. Finally, we used a magnet to separate the iron filings from the remaining mixture.
In conclusion, this lab successfully demonstrated the effectiveness of physical separation techniques in separating a mixture into its individual components. Filtration, magnetic separation, and evaporation proved to be reliable methods for separating the sand, iron filings, and salt respectively. The results obtained from this lab reinforced the importance of understanding the properties of each component in a mixture and devising appropriate separation techniques accordingly.