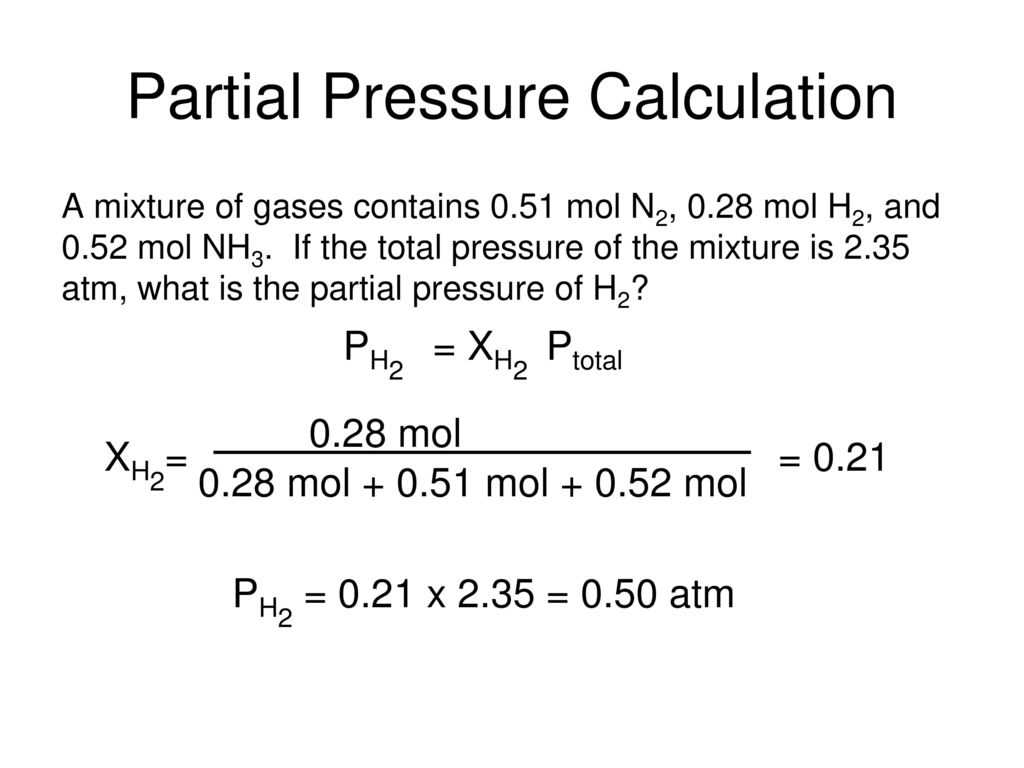
Understanding the concept of partial pressure is crucial in the study of gases, thermodynamics, and chemical reactions. When dealing with mixtures of gases, each gas exerts an individual pressure known as its partial pressure. To calculate the partial pressure of a gas, one must consider its mole fraction and the total pressure of the mixture.
However, working with partial pressures can be challenging, and students often encounter problems that require careful calculations and logical reasoning. These problems can involve various scenarios, such as ideal gases, Dalton’s law, and vapor pressure. To help you tackle these problems effectively, we have compiled a comprehensive guide that includes a variety of partial pressure problems and their corresponding answers.
The guide covers topics such as calculating partial pressure, finding mole fractions, determining total pressure, and applying Dalton’s law to mixtures. Each problem is explained step-by-step, with clear explanations and proper calculations. Additionally, the answers provided will allow you to verify your solutions and gain confidence in your understanding of partial pressure concepts.
Whether you are a student preparing for an exam or a curious mind seeking a deeper understanding of partial pressure, this guide will serve as a valuable resource. By practicing these problems and understanding the answers, you will enhance your problem-solving skills, reinforce key concepts, and develop a solid foundation in partial pressure calculations.
Partial Pressure Problems and Answers
Partial pressure refers to the pressure exerted by a single component in a mixture of gases. It is an important concept in understanding gas behavior and can be calculated using the ideal gas law. This concept is particularly relevant in various fields like chemistry, physics, and engineering, where gas mixtures and their properties are studied and analyzed.
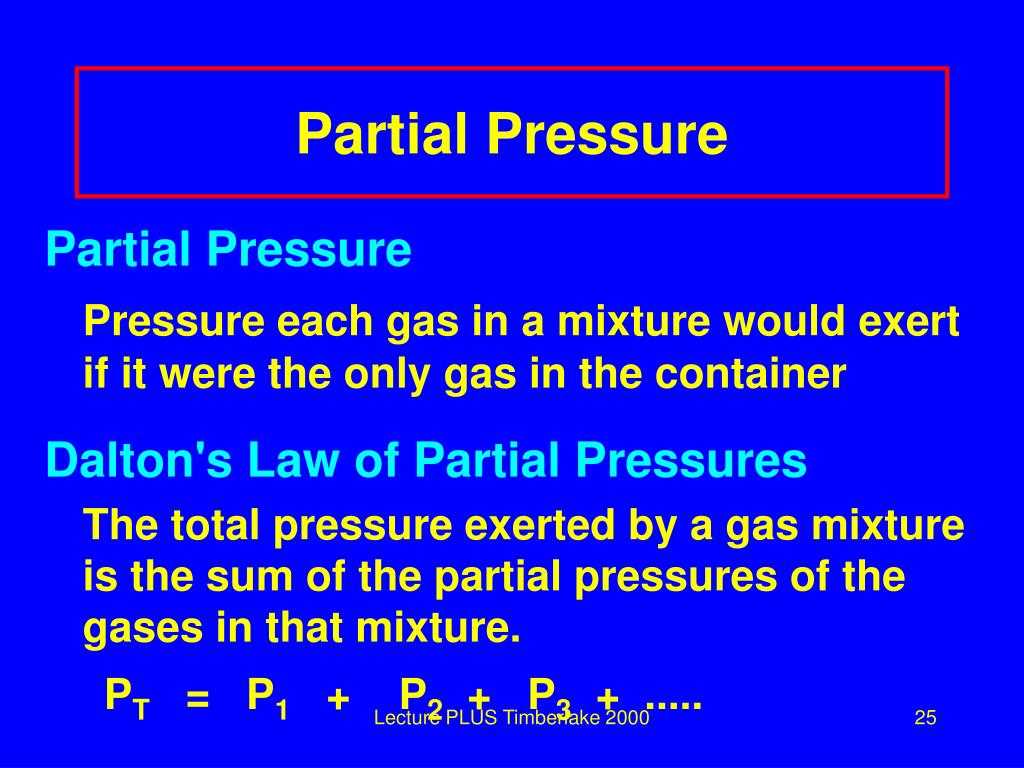
When solving partial pressure problems, it is essential to understand the relationship between the total pressure of a gas mixture and the partial pressures of its components. The total pressure is the sum of the partial pressures, where each partial pressure represents the contribution of a specific gas in the mixture. This relationship can be expressed mathematically using Dalton’s law of partial pressures.
To solve partial pressure problems, one needs to follow a systematic approach. First, identify the given information regarding the gas mixture, including the total pressure and the mole fractions or concentrations of each gas component. Then, calculate the moles or volumes of each gas based on the given data. Finally, apply the ideal gas law or Dalton’s law to determine the partial pressures of each gas component.
Having a clear understanding of the calculations involved in partial pressure problems is crucial. It helps in accurately determining the contribution of each gas component to the overall pressure of the mixture. Practicing such problems with various scenarios and gases can further enhance one’s problem-solving skills and comprehension of gas behavior.
Understanding Partial Pressure Equations
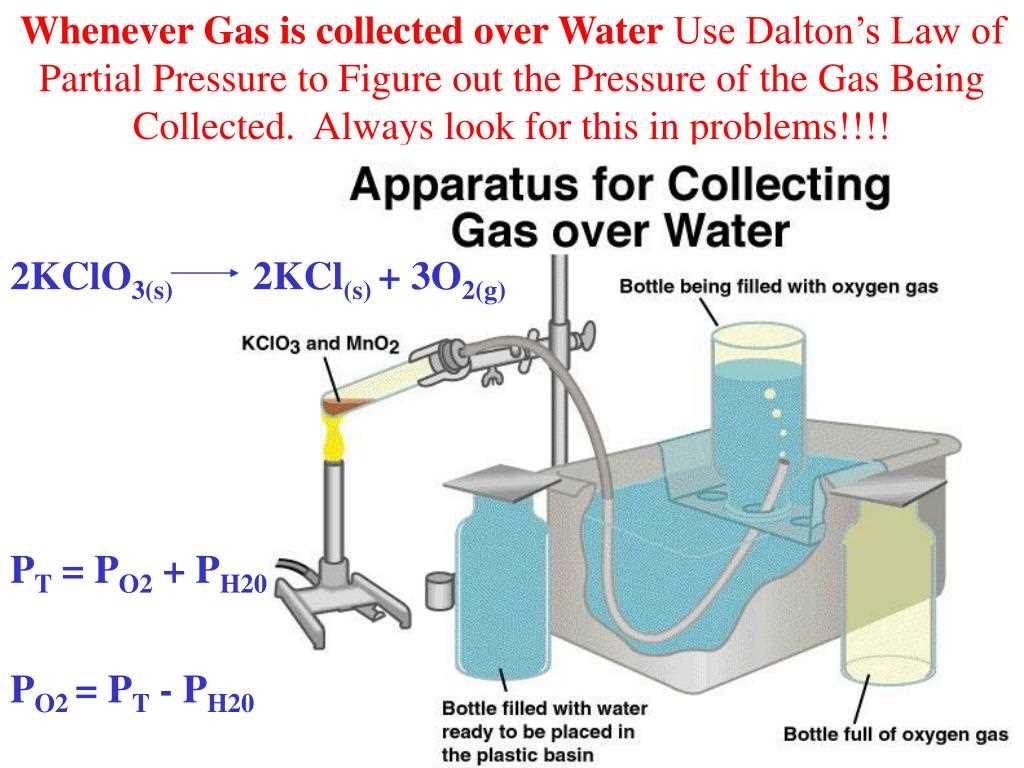
Partial pressure is a term used in chemistry to describe the pressure exerted by a single component of a gaseous mixture. It is an important concept when studying mixtures of gases, as it allows scientists to determine the individual contributions of each gas to the total pressure. The partial pressure of a gas can be calculated using the ideal gas law, which relates the pressure, volume, and temperature of a gas.
The ideal gas law is often written as PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin. To calculate the partial pressure of a gas in a mixture, we need to know the total pressure and the mole fraction of the gas.
The mole fraction of a gas is the ratio of the number of moles of that gas to the total number of moles in the mixture. It can be calculated using the equation X = n / n(total), where X is the mole fraction, n is the number of moles of the gas, and n(total) is the total number of moles in the mixture. Once we know the mole fraction, we can use it to calculate the partial pressure of the gas using the equation P(gas) = X(gas) * P(total), where P(gas) is the partial pressure of the gas and P(total) is the total pressure of the mixture.
Understanding partial pressure equations is important in various applications, such as in the pharmaceutical industry, where the partial pressure of gas may affect the solubility of a drug in a liquid. By controlling the partial pressures of different gases, scientists can optimize the formulation and stability of medications. Additionally, in environmental chemistry, understanding partial pressure equations allows researchers to study the behavior of gases in the atmosphere and predict their impact on climate change. Overall, the concept of partial pressure plays a crucial role in understanding the behavior of mixtures of gases and has implications in various scientific fields.
Calculating Partial Pressure
Partial pressure is a concept used in chemistry to describe the pressure exerted by a particular gas in a mixture. It is particularly useful in gases that consist of multiple components. To calculate the partial pressure of a gas, you need to know the mole fraction of the gas and the total pressure of the mixture. The mole fraction is the ratio of the number of moles of the specific gas to the total number of moles in the mixture.
To calculate the partial pressure, you can use the formula:
Partial Pressure = Mole Fraction × Total Pressure
For example, let’s say you have a mixture of gases that consists of oxygen and nitrogen. The mole fraction of oxygen is 0.40 and the total pressure of the mixture is 1.2 atm. To calculate the partial pressure of oxygen, you can use the formula:
Partial Pressure of Oxygen = 0.40 (mole fraction) × 1.2 atm (total pressure) = 0.48 atm
This means that the partial pressure of oxygen in the mixture is 0.48 atm. Similarly, you can calculate the partial pressure of nitrogen by using its mole fraction and the total pressure of the mixture.
By calculating the partial pressure of each gas in a mixture, you can determine the individual contributions of each gas to the total pressure. This is useful in many applications, such as understanding gas behavior, determining gas concentrations, and predicting the behavior of gas mixtures in different conditions.
Common Partial Pressure Problems
In chemistry, partial pressure refers to the pressure exerted by a single gas in a mixture of gases. This concept is often used in various calculations and problems to determine the behavior and properties of gases. Here are some common types of partial pressure problems that students may encounter:
Determining the Partial Pressure of a Gas
One common type of problem involves calculating the partial pressure of a specific gas in a mixture. To solve this type of problem, you need to know the total pressure of the mixture and the mole fraction or the partial pressure of the other gases present. By using Dalton’s law of partial pressures, you can calculate the partial pressure of the desired gas by subtracting the sum of the partial pressures of the other gases from the total pressure.
Calculating the Total Pressure of a Mixture
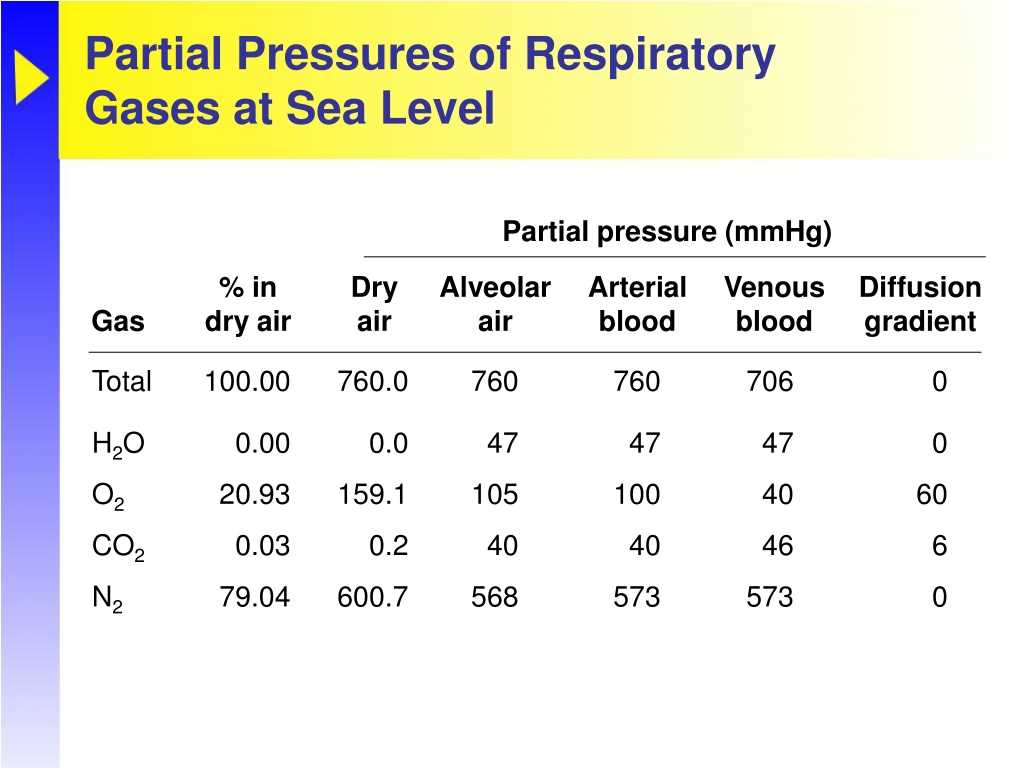
Another common partial pressure problem involves determining the total pressure of a gas mixture. This type of problem often requires you to know the mole fraction or the partial pressure of each gas in the mixture. By applying Dalton’s law of partial pressures, you can sum up the partial pressures of all the gases to find the total pressure of the mixture.
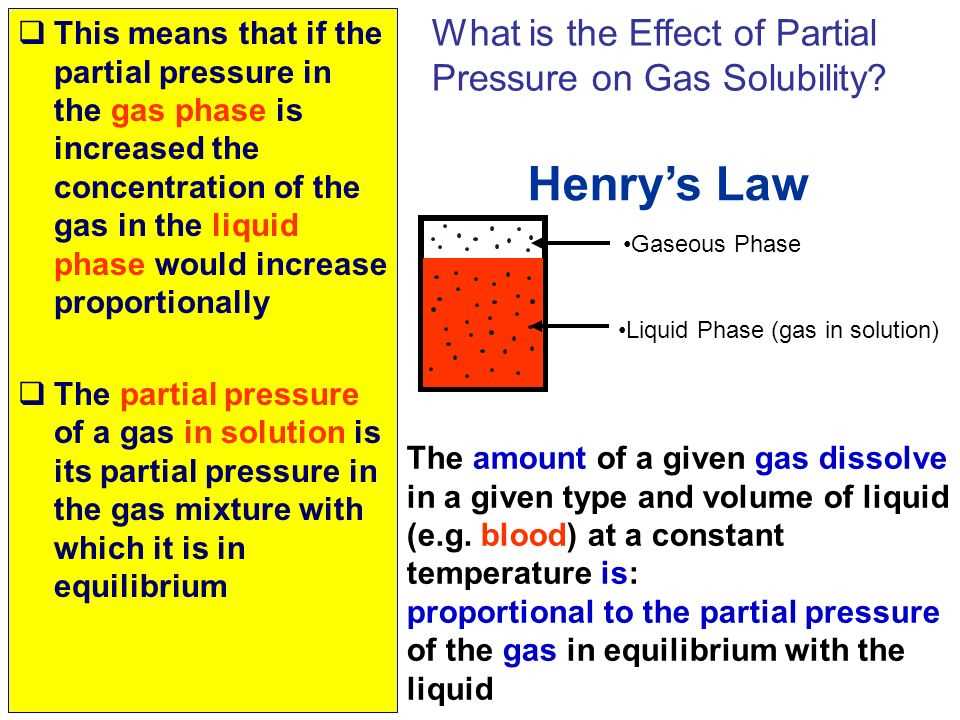
Using Partial Pressure to Calculate Solubility
Partial pressure can also be used to calculate the solubility of a gas in a liquid. In this type of problem, you need to know the partial pressure of the gas above the liquid and its solubility coefficient. By using Henry’s law, you can determine the solubility of the gas in the liquid based on the partial pressure.
These are just a few examples of the common types of partial pressure problems encountered in chemistry. By understanding the fundamentals and applying the appropriate formulas, you can successfully solve these problems and gain a deeper understanding of the behavior of gases.
Tips and Strategies for Solving Partial Pressure Problems
When it comes to solving partial pressure problems, there are several tips and strategies that can help you navigate through the calculations with ease. Understanding the concept of partial pressure and knowing the relevant equations is key to solving these types of problems. Here are some tips to consider:
- Identify the gases: Start by identifying all the gases present in the system. This will help you determine the total pressure and the partial pressures of each gas.
- Apply Dalton’s law: Dalton’s law states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. Use this law to calculate the partial pressures of the individual gases.
- Convert units: Make sure all the units are consistent in your calculations. Convert any given pressures or volumes to the appropriate units before proceeding with the calculations.
- Use mole fractions: Mole fractions are often used to express the concentration of gases in a mixture. They can be calculated by dividing the moles of a specific gas by the total moles of all the gases in the system.
- Consider the equilibrium: If the partial pressures are given at equilibrium, take into account any changes that occur during the reaction. This may require you to calculate the change in partial pressure before determining the final equilibrium values.
By following these tips and strategies, you can approach partial pressure problems with confidence. Take your time to understand the concepts, analyze the given information, and apply the appropriate equations. Practice solving various types of problems to improve your skills and become proficient in solving partial pressure problems.
Examples and Solutions with Partial Pressure
In this article, we have explored the concept of partial pressure and its significance in various fields such as chemistry and thermodynamics. We have discussed the definition of partial pressure and how it relates to the total pressure of a mixture of gases. We have also explored the relationship between partial pressure and the mole fraction of a gas component in a mixture.
To solidify our understanding of partial pressure, let’s go through a few examples and their solutions:
Example 1:
A mixture of gases contains oxygen (O2), nitrogen (N2), and carbon dioxide (CO2). The partial pressures of these gases are given as 300 torr, 400 torr, and 100 torr, respectively. Calculate the total pressure of the mixture.
Solution:
To find the total pressure, we simply add up the partial pressures of each gas component:
- Partial pressure of O2 = 300 torr
- Partial pressure of N2 = 400 torr
- Partial pressure of CO2 = 100 torr
Total pressure = 300 torr + 400 torr + 100 torr = 800 torr
Example 2:
A mixture of gases contains hydrogen (H2) and helium (He). The mole fractions of hydrogen and helium are 0.75 and 0.25, respectively. The total pressure of the mixture is 2.5 atm. Calculate the partial pressures of hydrogen and helium.
Solution:
To find the partial pressures, we can use the formula:
Partial pressure = Mole fraction × Total pressure
- Partial pressure of hydrogen = 0.75 × 2.5 atm = 1.875 atm
- Partial pressure of helium = 0.25 × 2.5 atm = 0.625 atm
Summary
Partial pressure is a significant concept in the study of gases. It allows us to analyze the contribution of each gas component in a mixture to the total pressure. By understanding the relationship between partial pressure and mole fraction, we can calculate the partial pressures of different gas components in a mixture. These calculations are crucial in various scientific and industrial applications, such as gas analysis and chemical reactions.