Understanding solubility is essential in the field of chemistry. Solubility is defined as the ability of a substance to dissolve in a solvent. It is usually expressed in terms of grams of solute that can dissolve in 100 grams of solvent at a specific temperature. Different substances have different solubilities, and studying solubility curves can provide valuable information about the behavior of these substances.
A solubility curve is a graphical representation of the relationship between the solubility of a compound and its temperature. It helps us understand how the solubility changes as the temperature varies. Solubility curves are typically generated by conducting experiments in which the solubility of a solute is determined at various temperatures. These experimental data points are then plotted on a graph, and a curve is drawn through the points to show the solubility trend.
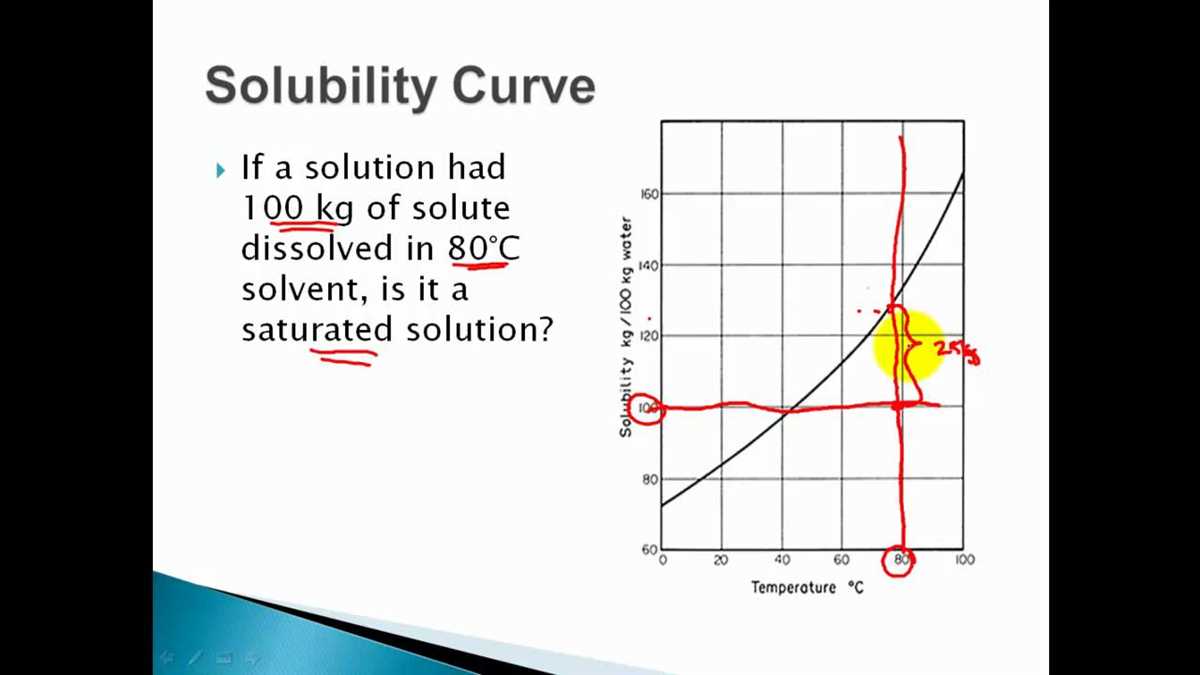
In order to interpret a solubility curve, it is important to understand what the various parts of the curve represent. The points on the curve represent the maximum amount of solute that can dissolve in the solvent at a specific temperature. Any point above the curve indicates that the solution is supersaturated, meaning that additional solute will not dissolve. Any point below the curve indicates that the solution is unsaturated, meaning that more solute can be dissolved.
The shape of a solubility curve can also provide valuable information. A curve that slopes upwards from left to right indicates that the solubility of the compound increases as the temperature increases. This is typical for most solid solutes in water. On the other hand, a curve that slopes downwards from left to right suggests that the solubility of the compound decreases as the temperature increases. This is typical for gases dissolved in water.
Solubility Curves Answer Key: Everything You Need to Know
If you are studying solubility curves, it is important to have an answer key to guide you through the process. Solubility curves represent the relationship between the solubility of a substance and temperature. This information is crucial for understanding how different substances dissolve and interact with each other.
With a solubility curves answer key, you can easily determine the solubility of a substance at any given temperature. The key provides a reference point for comparing the solubility of different substances. It allows you to identify trends and patterns in solubility, such as how solubility increases with temperature for some substances or decreases for others.
Key Information:
- The solubility of a substance is defined as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature.
- Solubility curves are typically graphed as a line or curve on a temperature vs. solubility graph.
- The solubility of a substance can be affected by factors such as pressure, concentration, and the presence of other substances (such as a common ion).
- Using the solubility curves answer key, you can predict the amount of solute that will dissolve at a certain temperature or determine the temperature at which a certain amount of solute will dissolve.
- Understanding solubility curves is particularly important in industries such as pharmaceuticals, where knowledge of solubility is crucial for drug formulation.
Overall, having access to a solubility curves answer key can greatly enhance your understanding of solubility and its relationship to temperature. It allows you to make predictions and draw conclusions about solubility, based on the provided data. So, whether you’re a student studying chemistry or a professional in the pharmaceutical industry, an answer key for solubility curves is a valuable tool to have.
Understanding Solubility Curves

The solubility of a substance refers to its ability to dissolve in a given solvent. Solubility curves are graphical representations of the solubility of a substance at different temperatures. These curves provide important information about the relationship between temperature and solubility and are commonly used in chemistry to predict the solubility of substances under various conditions.
To understand solubility curves, it is important to know that solubility is typically expressed in terms of grams of solute per 100 grams of solvent. The solubility curves plot these solubility values on a graph, with temperature on the x-axis and solubility on the y-axis. The curves may take different shapes depending on the nature of the substance being dissolved.
Key concepts:
- Saturated solution: A solution that contains the maximum amount of solute at a given temperature.
- Supersaturated solution: A solution that contains more solute than is normally possible at a given temperature.
- Unsaturated solution: A solution that contains less solute than could be dissolved at a given temperature.
By analyzing solubility curves, scientists can determine the solubility of a substance under specific conditions. For example, if the temperature is known, the solubility curve can be used to find the maximum amount of solute that can dissolve in a solvent. Similarly, if the amount of solute is known, the solubility curve can be used to estimate the temperature at which the solution will become saturated.
| Temperature (°C) | Solubility (g/100g H2O) |
|---|---|
| 0 | 50 |
| 10 | 60 |
| 20 | 70 |
| 30 | 80 |
In the example above, the solubility of Substance X in water increases as the temperature increases. At 0°C, the saturated solution can contain 50 grams of Substance X per 100 grams of water. As the temperature rises to 30°C, the solubility increases to 80 grams of Substance X per 100 grams of water.
Understanding solubility curves allows scientists to make informed predictions about the behavior of substances in solutions. These curves provide valuable insights into the solubility characteristics of various substances and are vital tools in the field of chemistry.
Factors Affecting Solubility
Solubility is the ability of a substance to dissolve in a solvent and form a homogeneous solution. It is influenced by several factors, including temperature, pressure, and the nature of the solute and solvent. Understanding these factors is essential for predicting and controlling solubility in various scenarios.
1. Temperature
Temperature plays a significant role in determining solubility. Generally, an increase in temperature leads to an increase in solubility for most solid solutes in liquid solvents. This is because higher temperatures provide more energy for the particles, allowing them to overcome the intermolecular forces and mix more readily. However, there are exceptions to this trend, such as the solubility of certain gases, which decreases with increasing temperature.
2. Pressure
Pressure affects the solubility of gases in liquids. According to Henry’s law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. As pressure increases, more gas molecules are forced into the liquid, resulting in higher solubility. Conversely, decreasing pressure causes the gas to escape from the liquid, reducing solubility.
3. Nature of the Solute and Solvent
The nature of the solute and solvent influences solubility. Polar solutes tend to dissolve better in polar solvents, while nonpolar solutes dissolve more readily in nonpolar solvents. This is due to the similar intermolecular forces between the solute and solvent molecules, allowing them to attract and mix more easily. Additionally, the size and shape of the solute and solvent molecules can impact solubility, as larger molecules may have difficulty fitting into the spaces between solvent molecules.
In conclusion, solubility is influenced by temperature, pressure, and the nature of the solute and solvent. Understanding these factors can help scientists and researchers predict and manipulate solubility in various applications, such as drug formulation, chemical reactions, and environmental processes.
Interpreting Solubility Curves
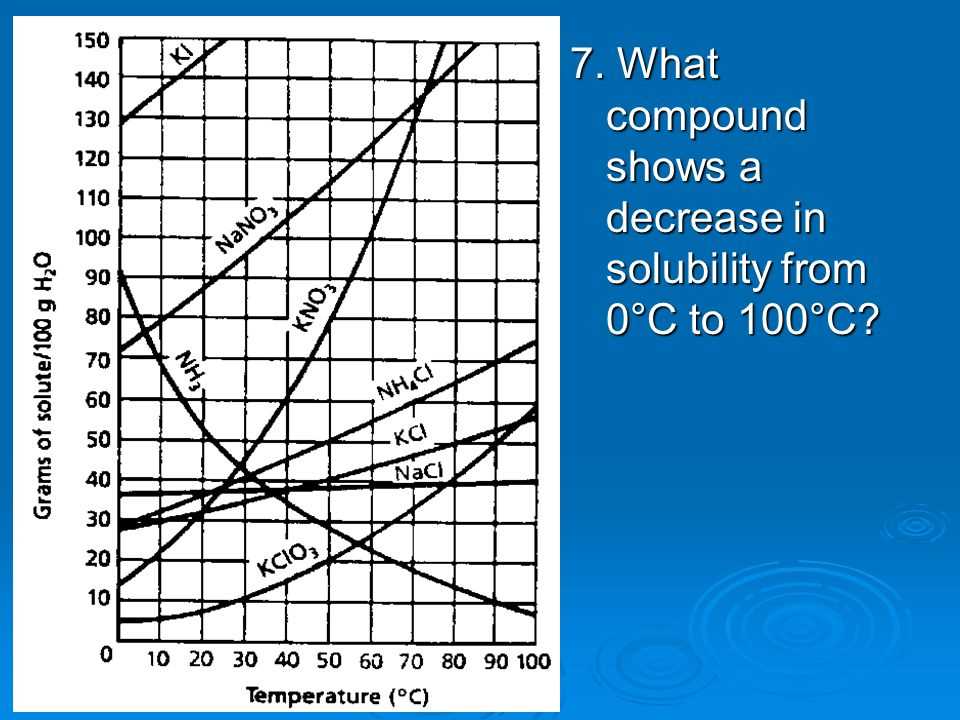
Solubility curves are graphical representations of how much solute can dissolve in a given amount of solvent at different temperatures. These curves provide valuable information about the solubility of different substances and can be used to determine the maximum amount of solute that can be dissolved in a given solvent at a specific temperature.
When interpreting solubility curves, it is important to understand that the solubility of a substance is affected by temperature. As the temperature increases, the solubility of most solid solutes also increases. This means that more solute can be dissolved in the solvent at higher temperatures. On the other hand, the solubility of most gases decreases as the temperature increases. This is because gases become less soluble in liquids as the temperature rises.
Key observations:
- The solubility curve for a specific substance shows how the solubility changes with temperature.
- If a point falls on the curve, it means that the solution is saturated at that temperature.
- If a point falls below the curve, it means that the solution is unsaturated and more solute can be dissolved at that temperature.
- If a point falls above the curve, it means that the solution is supersaturated and contains more solute than can normally be dissolved at that temperature.
Interpreting solubility curves can also help predict the outcomes of various chemical reactions. For example, if a solute is known to be highly soluble at a specific temperature, then mixing it with a solvent at that temperature will likely result in a homogeneous solution. Conversely, if the solute is known to be insoluble at a specific temperature, adding it to the solvent at that temperature will likely result in a heterogeneous mixture with the solute remaining as a solid.
Solubility Curves Worksheet
Solubility curves are used to represent the relationship between the solubility of a substance and temperature. In this worksheet, you will be analyzing solubility curves to answer questions about different substances and their solubility in water at various temperatures.
The worksheet consists of a series of solubility curves for different substances, each labeled with a specific temperature range. Based on these curves, you will be asked to determine the solubility of a substance at a given temperature, as well as identify the temperature at which a substance becomes saturated or begins to precipitate.
To complete the worksheet, you will need to interpret the information presented in the solubility curves. You will be required to compare the solubility of different substances at different temperatures and make predictions about the behavior of substances based on their solubility curves.
Additionally, the worksheet may include questions that require you to calculate the solubility of a substance at a specific temperature using the solubility curve as a reference. You may also be asked to analyze and interpret data from a solubility curve graphically or numerically.
This worksheet is designed to help you develop your understanding of solubility and how it changes with temperature. By analyzing and interpreting solubility curves, you will gain insights into the factors that influence solubility and how to predict the solubility of a substance at different temperatures.
Common Mistakes and Troubleshooting
When working with solubility curves, it is important to be aware of common mistakes that can occur and how to troubleshoot them. Here are some common mistakes and tips for troubleshooting:
1. Incorrectly reading the solubility curve: One common mistake is misreading the solubility curve, particularly when determining the solubility of a substance at a specific temperature. To avoid this, it is important to carefully follow the lines and data points on the curve. Double-checking the temperature and corresponding solubility values can help ensure accuracy.
2. Using the wrong units: Another frequent mistake is using the wrong units for temperature or solubility. Solubility curves often provide solubility values in grams of solute per 100 grams of solvent or per 100 mL of solvent, so it is important to pay attention to the units provided and use them correctly in calculations.
3. Incorrectly labeling the axes: One mistake that can lead to confusion is incorrectly labeling the axes on the solubility curve graph. It is crucial to correctly label the x-axis with temperature and the y-axis with solubility. Double-checking the labels can help prevent errors and ensure accurate interpretations of the data.
4. Missing data points: Sometimes, solubility curves may not provide data points at specific temperatures or concentrations. In such cases, it can be challenging to determine the solubility accurately. One possible solution is to estimate the solubility based on the trend of the curve. However, it is important to note that estimations may introduce some error into the results.
5. Variation in solubility due to impurities: Impurities in the solute or solvent can affect solubility and lead to deviations from expected values. If there are unexpected discrepancies between the observed solubility and the solubility indicated by the solubility curve, it may be worth investigating the purity of the substances used. Purifying the solute or solvent may help eliminate this source of error.
6. Using an outdated or inaccurate solubility curve: Finally, it is essential to ensure that the solubility curve being used is up to date and accurate for the specific solute and solvent being studied. Different substances may have different solubilities, so using a solubility curve that does not apply to the particular system can lead to incorrect results. Checking the source and reliability of the solubility curve is crucial to avoid this mistake.
Importance and Applications of Solubility Curves
Solubility curves are an essential tool in understanding the behavior of different substances and their ability to dissolve in a solvent. The information provided by solubility curves is not only important in scientific research but also has various practical applications in different fields.
1. Chemical Industry: Solubility curves are crucial in determining the feasibility of chemical reactions and the optimal conditions for producing a desired product. By understanding the solubility characteristics of different compounds, chemists can design processes that maximize the production yield and minimize waste.
2. Pharmaceutical Industry: Solubility is a critical factor in drug development as it affects the bioavailability and efficacy of medications. Solubility curves help pharmaceutical scientists determine how to formulate drugs for optimal absorption and delivery in the body. It also aids in identifying potential drug-drug interactions and determining the stability of drug formulations.
3. Environmental Analysis: Solubility curves play a vital role in determining the fate and transport of contaminants in natural environments, such as groundwater and surface water. By understanding the solubility of pollutants, scientists can assess the risk and impact of these substances on ecosystems and develop appropriate remediation strategies.
4. Agriculture: The solubility of fertilizers and pesticides is essential in determining their effectiveness and potential environmental impact. Solubility curves help farmers and agronomists understand how these substances behave in the soil and how they can be optimally applied to ensure crop health and minimize environmental damage.
5. Education: Solubility curves are a fundamental topic in chemistry education. Learning about solubility rules and interpreting solubility curves helps students develop critical thinking and problem-solving skills. Understanding solubility also lays the foundation for more advanced concepts in chemistry.
In conclusion, solubility curves play a crucial role in scientific research and have numerous practical applications in various industries. By providing insights into the behavior of substances in different solvents, solubility curves help in developing efficient processes, formulating effective medications, assessing environmental risks, optimizing agricultural practices, and fostering scientific education.