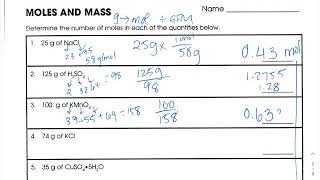
If you are studying chemistry and working with mole-mass conversions, then you might find yourself in need of some answers to practice problems. This worksheet provides those answers, so you can check your work and ensure that you’re on track with your understanding of this important concept.
The worksheet includes a variety of problems that ask you to convert between moles and mass, and vice versa. You will need to use the molar mass of each substance to complete these conversions. By practicing these problems and checking your answers with this worksheet, you can reinforce your understanding of how to perform mole-mass conversions and build your confidence in tackling more complex problems in the future.
Having access to the answers for these practice problems is crucial for self-study purposes. It allows you to check your work and identify any mistakes you may have made, so you can learn from them and improve. Additionally, seeing the correct answers can help you understand the steps and processes involved in mole-mass conversions, making it easier for you to apply these concepts to different types of problems.
Mole Mass Conversions Worksheet Answers
In the field of chemistry, mole mass conversions are an important topic to understand. This conversion allows scientists to determine the mass of a substance based on its molecular weight and the number of moles present. By using the mole mass conversions worksheet, students can practice solving problems and gain a better understanding of this concept.
The mole mass conversions worksheet provides a series of exercises that require students to convert between moles and grams. Students are given the molecular weight of a substance and must use this information to calculate the mass in grams. Similarly, they are given the mass of a substance and must determine the number of moles present.
To solve these problems, students can use the formula: Mass (in grams) = moles x molecular weight. By rearranging this formula, they can also solve for moles by dividing the mass (in grams) by the molecular weight. This worksheet helps students to practice applying this formula and strengthen their understanding of mole mass conversions.
The answers to the mole mass conversions worksheet are provided to help students check their work and verify their calculations. Each answer is accompanied by a step-by-step explanation of how it was derived, allowing students to understand the process and learn from any mistakes they may have made. This feedback is crucial for students to improve their problem-solving skills and develop a deeper understanding of mole mass conversions.
In conclusion, the mole mass conversions worksheet answers serve as a valuable resource for students studying chemistry. By practicing these conversions, students can strengthen their ability to convert between moles and grams, enhancing their understanding of this important concept in chemistry.
Understanding Molar Mass
The concept of molar mass is fundamental in chemistry as it allows us to calculate the mass of a substance on a molecular level. Molar mass is defined as the mass of one mole of a substance, and it is expressed in grams per mole (g/mol). By knowing the molar mass of a substance, we can determine the amount of that substance in moles, as well as its mass in grams.
Molar mass is calculated by summing the atomic masses of all the atoms in a molecule. Atomic masses are listed on the periodic table and are expressed in atomic mass units (amu). When calculating the molar mass of a compound, we multiply the atomic mass of each element by the number of atoms of that element in the molecule and then add these values together.
For example: The molar mass of water (H2O) can be calculated by adding the atomic masses of two hydrogen atoms (2 x 1.008 amu) and one oxygen atom (1 x 16.00 amu). The molar mass of water is therefore 18.02 g/mol.
Molar mass is a crucial concept in chemical calculations because it allows us to convert between mass and moles of a substance. This conversion is useful in a variety of applications, such as determining the amount of reactants needed for a chemical reaction, calculating the yield of a reaction, or determining the concentration of a solution.
In conclusion, understanding molar mass is essential in chemistry as it enables us to relate the mass of a substance to its molecular composition. By knowing the molar mass, we can perform conversions between moles and mass, which are often necessary in chemical calculations.
Converting Moles to Grams
A fundamental concept in chemistry is the conversion between moles and grams. This conversion is necessary when dealing with chemical equations and stoichiometry calculations. To convert moles to grams, one must first know the molar mass of the substance in question. Molar mass is the mass of one mole of a substance and is expressed in grams per mole. It is calculated by summing the atomic masses of all the atoms in the chemical formula.
To convert moles to grams, one can use the formula:
Grams = Moles × Molar Mass
This formula allows you to calculate the mass of a substance based on its moles. For example, if you have 2 moles of water (H2O), you can multiply it by the molar mass of water (18.01528 g/mol) to find that it weighs approximately 36.03 grams.
- Example:
- 2 moles H2O × 18.01528 g/mol = 36.03 grams
Converting moles to grams is a crucial step in various chemistry calculations. It allows chemists to determine the mass of a substance present in a given amount and vice versa. This conversion is vital in determining the quantities of reactants and products in chemical reactions, aiding in the understanding and prediction of chemical processes.
Converting Grams to Moles
The process of converting grams to moles involves using the molar mass of a substance. The molar mass is the mass of one mole of a substance, and it is expressed in grams per mole. To convert grams to moles, you need to divide the given mass of the substance by its molar mass.
Here is an example to illustrate the conversion process. Let’s say we have 24 grams of carbon dioxide (CO2). To convert this mass to moles, we first need to find the molar mass of CO2. The molar mass of carbon (C) is 12.01 grams per mole, and the molar mass of oxygen (O) is 16.00 grams per mole. Since CO2 has one carbon atom and two oxygen atoms, we can calculate its molar mass:
| Element | Atomic Mass (g/mol) | Number of Atoms | Subtotal |
|---|---|---|---|
| C | 12.01 | 1 | 12.01 |
| O | 16.00 | 2 | 32.00 |
| Total | – | – | 44.01 |
Now that we know the molar mass of CO2 is 44.01 grams per mole, we can calculate the number of moles in 24 grams of CO2. Using the formula:
moles = mass (grams) / molar mass (grams per mole)
We can plug in the values:
moles = 24 grams / 44.01 grams per mole ≈ 0.545 moles
Therefore, 24 grams of carbon dioxide is approximately equal to 0.545 moles.
This conversion process can be applied to any substance, as long as you know its molar mass. It is an essential step in many chemical calculations, such as determining the amount of reactants or products in a chemical reaction.
Calculating the Number of Moles
The concept of moles is fundamental in chemistry and is used to measure the amount of a substance. A mole represents a specific number of particles, which is 6.022 x 10^23. Knowing the number of moles provides valuable information about the quantity of a substance.
To calculate the number of moles, one must have the mass of the substance. The mass is usually given in grams or kilograms. Using the molar mass of the substance, which is the mass of one mole of the substance, the number of moles can be determined by dividing the mass by the molar mass.
Example:
Let’s say we have 25 grams of water (H2O). The molar mass of water is approximately 18 grams/mol. To calculate the number of moles, we divide the mass by the molar mass:
Number of Moles = Mass of Water / Molar Mass of Water
Number of Moles = 25 g / 18 g/mol = 1.39 mol
In this example, we have determined that we have 1.39 moles of water.
Calculating the number of moles is an essential step in many chemical calculations, such as determining reaction yields, finding empirical formulas, and balancing chemical equations. By understanding how to calculate the number of moles, chemists can accurately measure and manipulate substances in various chemical processes.
Solving Mole-Mass Conversions
The concept of mole-mass conversions plays a crucial role in chemistry, as it allows us to relate the number of moles of a substance to its mass. This knowledge is valuable in various areas, including stoichiometry, molecular weight calculations, and determining the amount of reactants needed in a chemical reaction.
In order to solve mole-mass conversions, one must first understand the concept of a mole. A mole represents a quantity of a substance that contains Avogadro’s number of particles, which is approximately 6.022 × 10^23. This means that one mole of any substance contains the same number of atoms, molecules, ions, or any other particles, regardless of their size or mass.
To convert between moles and mass, one needs the molar mass of the substance, which is the mass of one mole of that substance. The molar mass is expressed in grams per mole (g/mol) and can be calculated by adding up the atomic masses of all the atoms in the chemical formula of the substance.
When solving a mole-mass conversion problem, the first step is to identify the given information, such as the number of moles or the mass of the substance. Then, using the molar mass, the given quantity can be converted to the desired unit. This can be done by setting up a conversion factor using the molar mass as the ratio between grams and moles. The unit of moles cancels out, leaving the desired unit of mass.
To solve the conversion problem, the given quantity is multiplied by the conversion factor. The result is the desired quantity in the desired unit.
Overall, solving mole-mass conversions is an essential skill in chemistry, allowing scientists to relate the number of moles of a substance to its mass. By understanding and applying the concept of molar mass, accurate calculations and conversions can be made for various chemical applications.
Practice Problems

In order to solidify your understanding of mole mass conversions, it is important to practice solving various problems. Below are several practice problems that will help you become more proficient in converting between moles and grams.
Problem 1:

Calculate the mass of 2 moles of oxygen gas (O2).
To solve this problem, we can use the molar mass of oxygen gas, which is 32 g/mol. To find the mass, we need to multiply the number of moles by the molar mass:
- 2 moles of O2 x 32 g/mol = 64 grams of O2
Problem 2:
Find the number of moles in 50 grams of sulfur dioxide (SO2).
To solve this problem, we can use the molar mass of sulfur dioxide, which is 64 g/mol. To find the number of moles, we need to divide the mass by the molar mass:
- 50 grams of SO2 / 64 g/mol = 0.78125 moles of SO2
Problem 3:
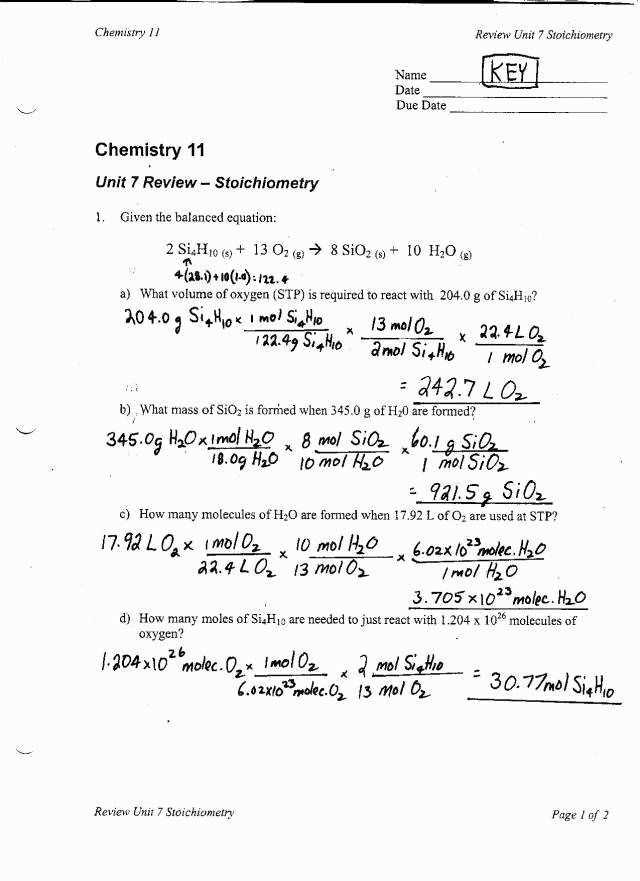
Convert 0.5 moles of water (H2O) to grams.
To solve this problem, we can use the molar mass of water, which is 18 g/mol. To find the mass, we need to multiply the number of moles by the molar mass:
- 0.5 moles of H2O x 18 g/mol = 9 grams of H2O
By practicing these types of problems, you will improve your ability to convert between moles and grams, which is an essential skill in chemistry. Additionally, it is important to understand the concept of molar mass and how it is used in these calculations. Remember to always double-check your work and use the correct units in your answers.
Answer Key
In the mole mass conversions worksheet, students were given a series of problems that required them to convert between the mass of a substance and its corresponding number of moles. The worksheet provided the students with the necessary information, such as the molar mass of the substance and the given mass. Using this information, students were able to apply the concept of moles and use the mole-to-mass or mass-to-mole conversions to find the desired answer.
The answer key for the mole mass conversions worksheet provides the correct solutions to each problem. It allows students to check their work and verify if they have correctly applied the conversion factors and calculations. The answer key serves as a guide for students to understand the correct approach and steps to solve the problems.
Below is an example of an answer key for one of the problems in the mole mass conversions worksheet:
| Given: | Mass of substance = 25.0 grams |
|---|---|
| Molar mass: | Substance X = 50.0 g/mol |
| To find: | Number of moles of Substance X |
| Solution: | Using the mole-to-mass conversion: |
| 25.0 g Substance X * (1 mol Substance X / 50.0 g Substance X) = 0.5 mol Substance X |
By providing the answer key, students can compare their own answers with the correct solutions and identify any mistakes made. It allows for self-assessment and helps students improve their understanding of mole mass conversions.