Chemical bonding is a fundamental concept in chemistry that helps us understand the behavior of atoms and molecules. It is the force that holds atoms together in a molecule and determines their arrangement in a compound. The study of chemical bonding is crucial for our understanding of various chemical processes, such as reactions, reactions, and the properties of materials.
Chemical bonding is a result of the interactions between the outermost electrons of atoms. These electrons are involved in bonding and are called valence electrons. The number of valence electrons determines an atom’s chemical properties and its ability to form bonds. The process of chemical bonding involves the redistribution of these valence electrons and the formation of new bonds between atoms.
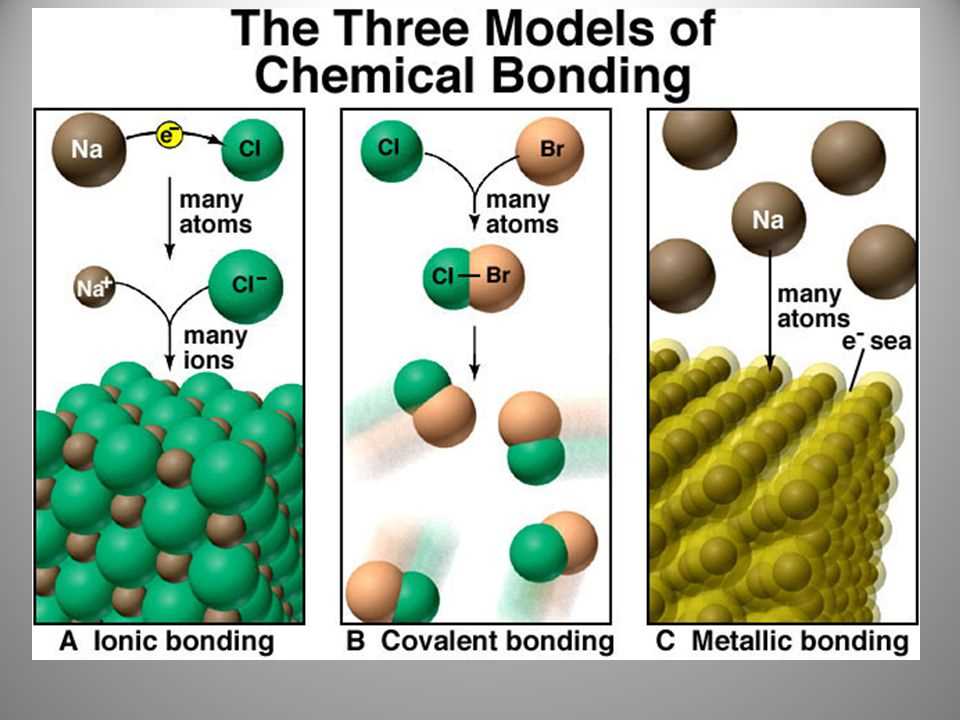
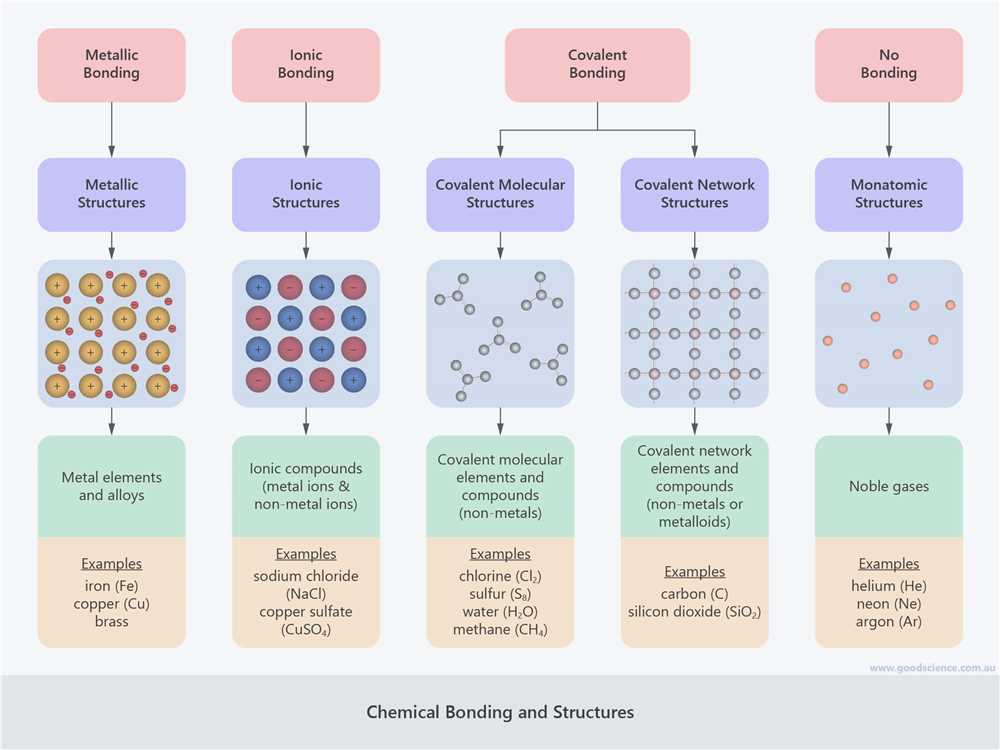
There are three primary types of chemical bonding: ionic bonding, covalent bonding, and metallic bonding. In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of ions. Covalent bonding occurs when atoms share electrons to achieve a stable electron configuration. Metallic bonding is unique to metals and involves a sea of delocalized electrons that are free to move throughout the material.
Understanding chemical bonding is essential for predicting the types of compounds that will form between different elements and explaining their physical and chemical properties. It allows us to explain phenomena such as the conductivity of metals, the crystalline structure of salts, and the properties of polymers. Additionally, chemical bonding plays a vital role in fields such as materials science, pharmaceuticals, and environmental science.
In this article, we will delve into the basics of chemical bonding, exploring the various types of bonds and their characteristics. We will also discuss key concepts and provide an answer key to commonly asked questions about chemical bonding.
What is Chemical Bonding?
Chemical bonding is the process by which atoms join together to form molecules or compounds. It is the fundamental concept in chemistry that explains how elements interact and combine with each other to create different substances. Understanding chemical bonding is crucial for understanding the properties and behavior of matter.
In chemical bonding, atoms share, donate, or receive electrons to achieve a stable electron configuration. The electrons in an atom are arranged in energy levels or shells, and the outermost shell, known as the valence shell, determines the chemical properties of the element. Atoms with incomplete valence shells tend to form chemical bonds in order to achieve greater stability.
There are different types of chemical bonds, including covalent bonds, ionic bonds, and metallic bonds. Covalent bonds occur when two atoms share electrons, resulting in the formation of molecules. Ionic bonds occur when one atom donates electrons to another atom, resulting in the formation of ions with opposite charges that attract each other. Metallic bonds occur when electrons are delocalized and shared among a lattice of metal atoms.
Chemical bonding plays a vital role in the formation and functioning of all living things. For example, the bonds between atoms in DNA molecules enable the transfer and storage of genetic information. Additionally, chemical bonding is responsible for the formation of complex structures, such as proteins and carbohydrates, which are essential for the functioning of cells and organisms.
Understanding chemical bonding allows scientists to predict the behavior and properties of substances, and it underlies many technological advancements. It is an essential concept in fields such as materials science, pharmacology, and environmental science. By studying chemical bonding, scientists can develop new materials, drugs, and technologies that have a wide range of applications in various industries.
Types of Chemical Bonds
In chemistry, chemical bonding is the process by which atoms combine to form molecules or compounds. There are several types of chemical bonds that can occur between atoms, each resulting in a different type of molecule. Understanding these different types of chemical bonds is important for understanding the properties and behavior of substances.
Covalent bonds are formed when atoms share electrons to form a stable electron configuration. In a covalent bond, the atoms involved have similar electronegativity, meaning they have similar tendencies to attract electrons. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms.
Ionic bonds are formed when electrons are transferred from one atom to another. This results in the formation of ions, which are charged particles. The positively charged ion is called a cation, and the negatively charged ion is called an anion. Ionic bonds are typically formed between a metal and a nonmetal.
Metallic bonds occur when electrons are delocalized and shared among a metal’s positively charged ions, known as cations. This results in a structure called a metallic lattice, where the cations are surrounded by a “sea” of freely moving electrons. Metallic bonds are responsible for the unique properties of metals, such as their high electrical and thermal conductivity.
Hydrogen bonds are a special type of weak bond that occurs between a hydrogen atom bonded to a highly electronegative atom, such as oxygen or nitrogen. These bonds result from the attraction between the partially positive hydrogen atom and the partially negative atom of a neighboring molecule. Hydrogen bonds play a crucial role in many biological processes, such as DNA replication and protein folding.
Overall, the different types of chemical bonds have a significant impact on the structure, properties, and behavior of substances. By understanding these bonds, scientists can predict and explain the properties of different compounds, as well as design new materials with specific properties.
Ionic Bonding: Key Features and Examples
In chemical bonding, ionic bonding is a type of bond that occurs between two atoms with significantly different electronegativity values. It involves the complete transfer of electrons from one atom to another, leading to the formation of ions. This results in the creation of an electrostatic attraction between the positively and negatively charged ions, known as an ionic bond.
The key features of ionic bonding include the formation of ions, the transfer of electrons, and the creation of an electrostatic attraction. In an ionic bond, the atom that loses electrons becomes a positively charged cation, while the atom that gains electrons becomes a negatively charged anion. The overall compound formed by the ionic bond is electrically neutral.
One example of ionic bonding is the formation of sodium chloride (NaCl). Sodium, which has a low electronegativity, loses one electron and becomes a sodium cation (Na+), while chlorine, which has a high electronegativity, gains one electron and becomes a chloride anion (Cl-). The electrostatic attraction between the sodium cation and chloride anion leads to the formation of ionic bonds in solid sodium chloride.
Another example is the formation of magnesium oxide (MgO). Magnesium, with a low electronegativity, loses two electrons and becomes a magnesium cation (Mg2+), while oxygen, with a high electronegativity, gains two electrons and becomes an oxide anion (O2-). The electrostatic attraction between the magnesium cation and oxide anion results in the formation of ionic bonds in solid magnesium oxide.
Covalent Bonding: Understanding the Sharing of Electrons
Covalent bonding is a fundamental concept in chemistry that involves the sharing of electrons between atoms. This type of bonding occurs between nonmetals and is characterized by the formation of covalent bonds, which are strong and directional. Understanding covalent bonding is essential to comprehend the behavior and properties of various molecules and compounds.
In a covalent bond, two atoms share one or more pairs of electrons. This shared electron pair is known as a bonding pair, and it is represented by a line or a dash between the atomic symbols. The electrons in the bonding pair are attracted to both nuclei, creating a strong bond that holds the atoms together. This sharing of electrons allows each atom to achieve a more stable electron configuration, typically the octet rule, where they have a full valence shell.
The strength of a covalent bond depends on the number of shared electrons and the electronegativity difference between the atoms involved. When the electronegativity difference is small, the bond is considered nonpolar covalent, meaning the electrons are shared equally. When the electronegativity difference is larger, the bond becomes polar covalent, where the electrons are shared unequally, resulting in a partial positive and partial negative charges.
Covalent bonding plays a crucial role in determining the physical and chemical properties of substances. It influences factors such as boiling and melting points, solubility, and reactivity. The sharing of electrons allows atoms to form stable molecules and compounds and enables the formation of complex structures like polymers. Understanding the nature and behavior of covalent bonds is essential for many fields, including organic chemistry, biochemistry, and materials science.
Overall, covalent bonding is a vital concept in chemistry that involves the sharing of electrons between atoms. It allows atoms to achieve a stable electron configuration and determines the behavior and properties of molecules and compounds. This type of bonding is strong, directional, and can be nonpolar or polar depending on the electronegativity difference between the atoms involved. Covalent bonding is a fundamental concept that forms the basis for understanding the chemical world around us.
Metallic Bonding: A Unique Bonding Model in Metals

Metallic bonding is a unique type of chemical bonding that occurs between metal atoms. It is different from ionic bonding and covalent bonding, which are the primary bonding models in non-metallic substances. Metallic bonding is responsible for the unique properties of metals, including their high thermal and electrical conductivity, malleability, and ductility.
In metallic bonding, the valence electrons of metal atoms are delocalized, meaning that they are not associated with a specific atom but rather move freely throughout the entire metal lattice. This delocalization of electrons creates a “sea” of electrons that surround the positively charged metal ions, forming a three-dimensional network. The metal ions themselves are held together by the electrostatic attraction between their positively charged nuclei and the negatively charged electron sea.
This electron sea model explains several key properties of metals. The delocalized electrons are able to move freely, allowing metals to conduct electricity and heat. The mobility of these electrons also gives metals their ability to be shaped and deformed without breaking, as the positive ions can slide past each other without disrupting the overall structure. This is why metals are malleable and ductile.
The strength of metallic bonding is determined by the number of valence electrons and the size of the metal ions. Metals with more valence electrons or smaller ions tend to have stronger metallic bonding. Additionally, the presence of alloying elements can affect the strength of metallic bonding. Alloying elements can disrupt the regular arrangement of metal ions and delocalized electrons, leading to changes in the properties of the metal.
In conclusion, metallic bonding is a unique bonding model that explains the properties and behavior of metals. It is characterized by the delocalization of valence electrons, creating a sea of electrons that surround the metal ions. This bonding model is responsible for the high conductivity, malleability, and ductility of metals, making them essential materials in various industries.
Intermolecular Forces: The Bonds Between Molecules
Intermolecular forces are the attractive forces that exist between molecules. While chemical bonds hold atoms together within a molecule, intermolecular forces are responsible for the interactions between molecules. These forces play a crucial role in determining the physical properties and behavior of substances, such as their boiling points, melting points, and solubilities.
There are three primary types of intermolecular forces: London dispersion forces, dipole-dipole forces, and hydrogen bonding. London dispersion forces are the weakest of the three and occur between all molecules, regardless of their polarity. These forces are due to temporary fluctuations in electron distribution that result in temporary dipoles, which can induce dipoles in neighboring molecules. Dipole-dipole forces, on the other hand, occur between polar molecules and involve the attraction between the positive end of one molecule and the negative end of another. Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and is attracted to an electronegative atom in a neighboring molecule.
The strength of intermolecular forces increases with increasing molecular size and polarity. Larger molecules have a greater number of electrons, which leads to greater temporary fluctuations in electron distribution and stronger London dispersion forces. Polar molecules, which have an uneven distribution of charge, experience stronger dipole-dipole forces compared to nonpolar molecules. Hydrogen bonding is the strongest type of intermolecular force and can significantly affect the physical and chemical properties of substances.
Summary:
- Intermolecular forces are the attractive forces that exist between molecules.
- There are three primary types of intermolecular forces: London dispersion forces, dipole-dipole forces, and hydrogen bonding.
- The strength of intermolecular forces increases with increasing molecular size and polarity.
Bonding Theories: Lewis Structures and Molecular Geometry
The study of chemical bonding is crucial in understanding the formation and behavior of molecules. Two important bonding theories, Lewis structures and molecular geometry, provide valuable insights into the arrangement of atoms in molecules and their properties.
Lewis structures are diagrams that represent the valence electrons of atoms in a molecule. They are based on the concept of octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In a Lewis structure, each valence electron is represented as a dot around the symbol of the atom. The electrons are then paired up to form bonds, either by sharing electrons (covalent bond) or by transferring electrons (ionic bond).
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. It is determined by the bonding pairs and lone pairs of electrons around the central atom. The shape of a molecule influences its polarity, reactivity, and physical properties. The VSEPR (valence shell electron pair repulsion) theory is commonly used to predict the molecular geometry. It states that electron pairs around the central atom will arrange themselves as far apart as possible to minimize repulsion, resulting in specific geometric shapes.
In summary, Lewis structures provide a visual representation of the bonding electrons in a molecule, while molecular geometry describes the arrangement of atoms in three-dimensional space. Understanding these concepts allows chemists to predict and explain the behavior of molecules, including their chemical reactivity and physical properties.