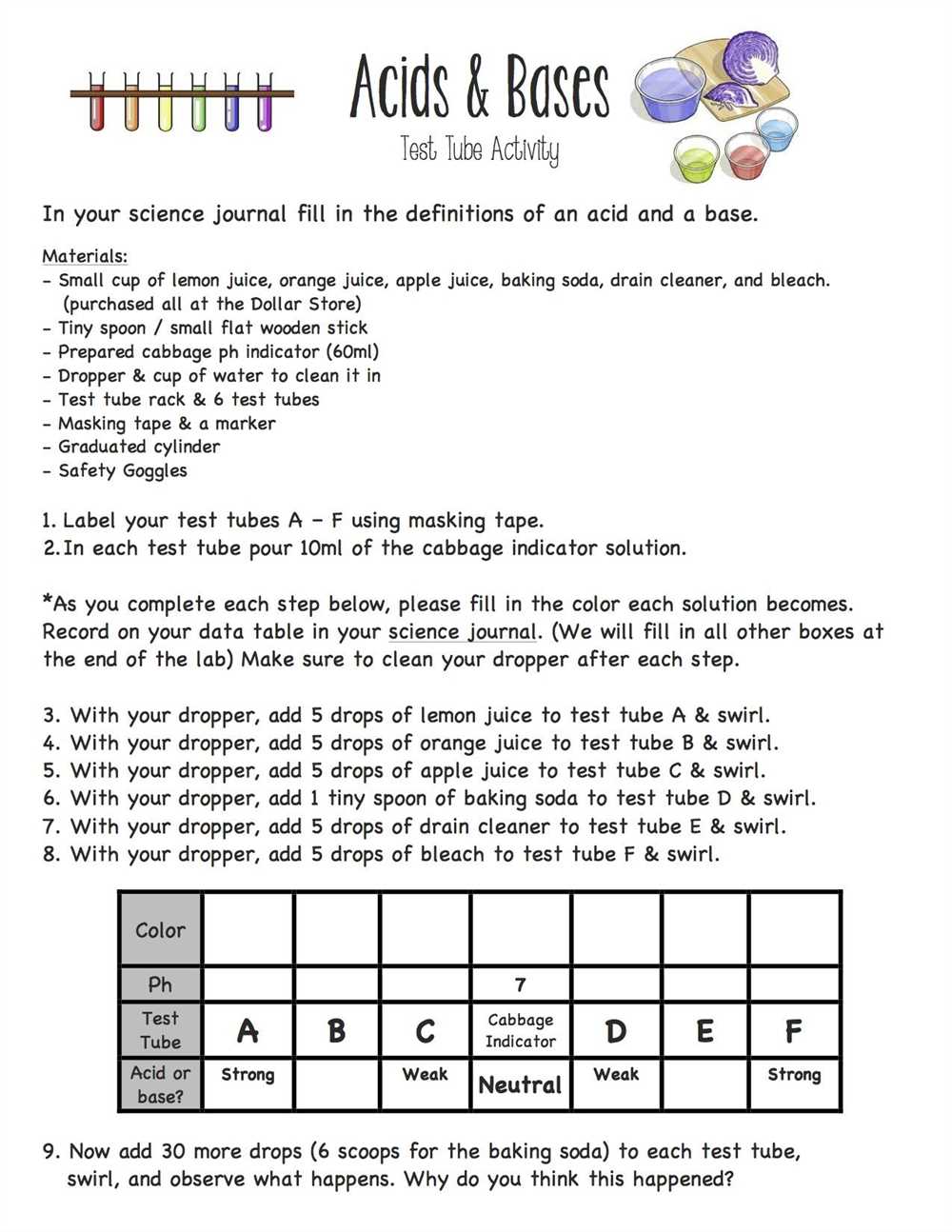
Understanding the concept of acids and bases in chemistry is essential for students to grasp the overall understanding of chemical reactions and how substances interact with one another. To reinforce this knowledge and test their understanding, teachers often assign worksheets to their students. However, without access to the correct answers, students may struggle to assess their progress and identify any areas of misunderstanding.
In this article, we provide the answers to a comprehensive acids and bases chemistry worksheet. By reviewing these answers, students can compare their own responses and correct any misunderstandings. This will enable them to enhance their understanding of acid-base reactions and strengthen their knowledge of key concepts, such as pH, acidity, and alkalinity.
Within the worksheet, students will encounter a variety of questions and scenarios that require a thorough understanding of acids and bases. The answers provided in this article not only explain the correct response, but also provide detailed explanations and examples to further deepen students’ comprehension.
By having access to the correct answers, students can receive immediate feedback on their performance and improve their overall understanding of acids and bases. This will ultimately enhance their chemistry skills and prepare them for more advanced topics in the future.
Acids and Bases Chemistry Worksheet Answers
When studying acids and bases in chemistry, it is important to have a solid understanding of their properties and behaviors. One way to test your knowledge on this topic is by completing a worksheet that provides various questions and scenarios related to acids and bases.
This worksheet typically includes questions on topics such as the definition of acids and bases, their properties, pH scale, neutralization reactions, and calculations involving molarity and volume. The answers to these questions can help reinforce your understanding of the concepts and principles discussed in class.
Here are some examples of possible answers you might find in an acids and bases chemistry worksheet:
- What is the definition of an acid? – An acid is a compound that donates a proton (H+) or accepts an electron pair in a chemical reaction.
- What are the properties of acids? – Acids typically have a sour taste, turn blue litmus paper red, and react with metals to produce hydrogen gas.
- What is the pH scale? – The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being highly acidic, 7 being neutral, and 14 being highly basic (alkaline).
- What is a neutralization reaction? – A neutralization reaction is a chemical reaction between an acid and a base that results in the formation of water and a salt.
- How do you calculate molarity? – Molarity (M) can be calculated by dividing the moles of solute by the volume of the solution in liters.
By reviewing the answers to these questions and practicing similar problems, you can strengthen your knowledge and skills in the field of acids and bases chemistry.
What Are Acids and Bases?
Acids and bases are two types of chemical substances that play crucial roles in various chemical reactions and processes. They can be found in everyday life, such as in the foods we eat and the cleaning products we use. Understanding the characteristics and properties of acids and bases is essential for understanding their effects on different materials and their impact on our environment.
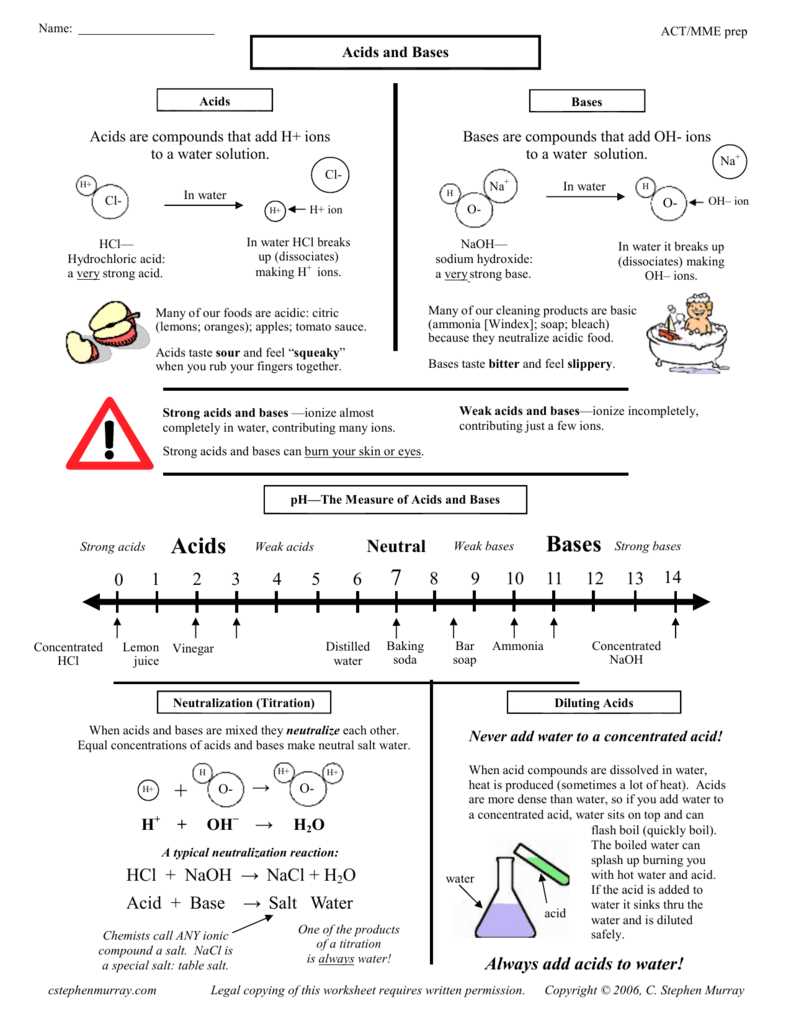
Acids are substances that can donate hydrogen ions (H+) in a chemical reaction. They have a sour taste, like vinegar or lemons, and can cause a burning or stinging sensation if they come into contact with the skin. Acids are known for their corrosive properties, as they can erode certain materials, such as metals and fabrics. Examples of common acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
Bases, on the other hand, are substances that can accept hydrogen ions (H+) or donate hydroxide ions (OH-) in a chemical reaction. They have a bitter taste and a slippery or soapy feel. Bases are known for their ability to neutralize acids, forming water and a salt. Common examples of bases include sodium hydroxide (NaOH) and ammonia (NH3).
Acids and bases can be classified based on their strength and concentration. Strong acids and bases completely dissociate in water, releasing all of their hydrogen ions or hydroxide ions. Weak acids and bases only partially dissociate, resulting in a lower concentration of hydrogen ions or hydroxide ions. The pH scale is commonly used to measure the acidity or alkalinity of a substance, with a pH of 7 considered neutral. Acids have a pH below 7, while bases have a pH above 7.
In summary, acids are substances that donate hydrogen ions, have a sour taste, and can erode materials, while bases are substances that accept hydrogen ions, have a bitter taste, and can neutralize acids. Understanding the properties and behavior of acids and bases is important in fields such as chemistry, biology, and environmental science.
Important Properties of Acids
Acids are a class of chemical compounds that have several important properties. These properties help to define and classify acids, as well as determine their behavior in chemical reactions.
One of the key properties of acids is their ability to release hydrogen ions (H+) when dissolved in water. This is known as the acidic nature of acids. The concentration of hydrogen ions determines the acidity of an acid, with higher concentrations resulting in stronger acids. Acidity can be measured using the pH scale, where values below 7 indicate acidity.
Another important property of acids is their ability to react with bases to form salts and water. This reaction is known as neutralization and is a common way to neutralize the effects of an acid. The reaction between an acid and a base is characterized by the transfer of hydrogen ions from the acid to the base, resulting in the formation of a salt. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products formed are sodium chloride (NaCl) and water (H₂O).
Acids also have a sour taste and can corrode metals. This corrosive property is due to their ability to react with metal ions, causing the metal to break down and form metal salts. The strength of acid’s corrosive properties depends on factors such as concentration and temperature.
- Acids can be divided into two main types – strong acids and weak acids. Strong acids fully dissociate in water, releasing all their hydrogen ions, while weak acids only partially dissociate.
- Common examples of acids include sulfuric acid (H₂SO₄), hydrochloric acid (HCl), and acetic acid (CH₃COOH).
In summary, acids have several important properties, including their ability to release hydrogen ions, react with bases to form salts, and corrode metals. These properties play a crucial role in understanding the behavior and applications of acids in chemistry.
Key Characteristics of Bases
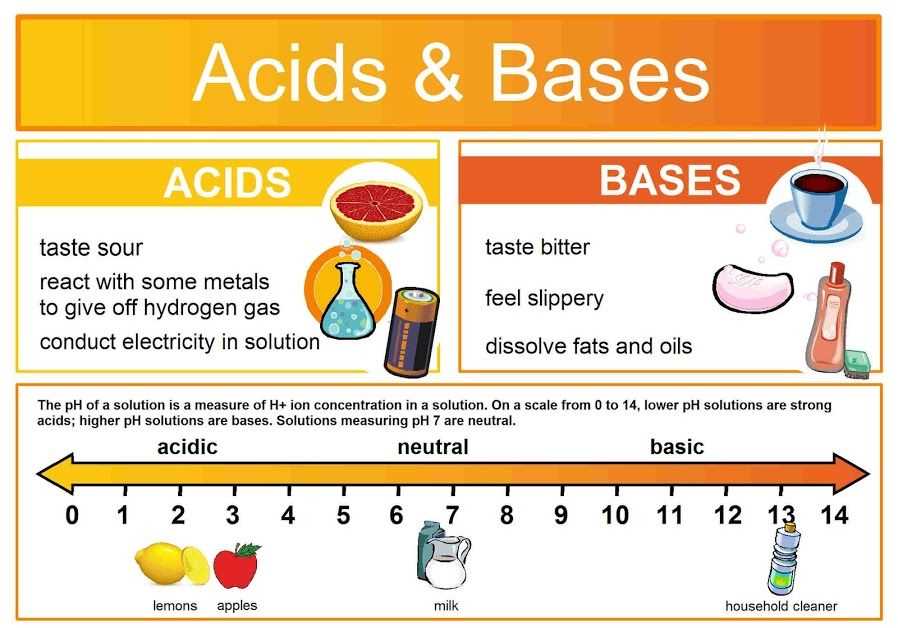
When studying acids and bases in chemistry, it is essential to understand the key characteristics that differentiate bases from acids. Bases are substances that have certain distinct qualities that set them apart from acids. These characteristics include:
- Arrhenius Definition: According to the Arrhenius definition, bases are substances that release hydroxide ions (OH-) in aqueous solution. This means that when a base is dissolved in water, it dissociates to form OH- ions.
- Bitter Taste: Bases have a characteristic bitter taste. This taste can be detected when tasting substances such as baking soda or soap.
- Slippery Texture: Bases have a slippery or soapy texture when touched. This can be observed when handling substances like lye or ammonia.
- pH: Bases have a pH greater than 7 on the pH scale. The pH scale measures the concentration of hydrogen ions in a solution, with lower pH values indicating higher acidity and higher pH values indicating alkalinity or basicity.
These key characteristics help identify bases and distinguish them from acids in various chemical reactions and reactions with indicators. Understanding these characteristics is crucial in the study and application of acids and bases in chemistry.
Definition and Examples of pH

pH is a measure of the acidity or alkalinity of a solution. It indicates the concentration of hydrogen ions (H+) present in a solution. pH is an important concept in chemistry and is commonly used to classify substances as acidic, neutral, or basic.
On a scale of 0 to 14, a pH of 7 is considered neutral, indicating that the solution is neither acidic nor basic. A pH below 7 indicates acidity, with a lower pH indicating a higher level of acidity. A pH above 7 indicates alkalinity, with a higher pH indicating a higher level of alkalinity.
A substance with a pH of 1 is highly acidic, while a substance with a pH of 14 is highly basic. Some examples of acidic substances include lemon juice, vinegar, and battery acid. Some examples of basic substances include baking soda, soap, and bleach. Water, with a pH of 7, is considered neutral.
pH is measured using a pH scale and a pH meter. The scale ranges from 0 to 14, with 7 being neutral. A pH meter measures the electrical potential difference between two electrodes immersed in the solution and gives a numerical value that represents the pH of the solution.
Understanding the pH of a solution is important in various fields, such as medicine, environmental science, and food science. It allows scientists and researchers to study the effects of acidity or alkalinity on biological processes, environmental conditions, and the properties of substances.
In summary, pH is a measure of the acidity or alkalinity of a solution and is determined by the concentration of hydrogen ions. It is used to classify substances as acidic, neutral, or basic and is measured on a scale of 0 to 14. pH has various applications in different fields and is an important concept in chemistry.
How to Calculate pH
pH is a measure of the acidity or alkalinity of a solution. It is important to calculate pH in various chemical and biological processes. pH is measured on a scale from 0 to 14, with 0 being highly acidic and 14 being highly alkaline. A pH of 7 is considered neutral. pH calculations involve the use of the concentration of hydrogen ions in a solution.
To calculate pH, the first step is to determine the concentration of hydrogen ions in the solution. This can be done by using the formula: pH = -log[H+]. Here, [H+] represents the concentration of hydrogen ions in moles per liter. The negative logarithm of the hydrogen ion concentration gives the pH value.
For example, let’s consider a solution with a hydrogen ion concentration of 1 x 10^-4 M. To calculate the pH, we can use the formula: pH = -log(1 x 10^-4) = 4. This means that the pH of the solution is 4, indicating acidity.
It’s important to note that the pH scale is logarithmic, meaning that each pH unit represents a tenfold change in acidity or alkalinity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4. Similarly, a solution with a pH of 1 is one hundred times more acidic than a solution with a pH of 3.
Calculating pH is an essential skill in understanding the properties and behavior of acids and bases. It allows chemists and scientists to determine the acidity or alkalinity of a solution and make informed decisions in various processes and experiments.
Acid-Base Reactions and Indicators
In chemistry, acid-base reactions play a crucial role in understanding the behavior of various substances. Acids and bases are important chemical species that can donate or accept protons, respectively. These reactions are essential for a wide range of applications, including industrial processes, environmental monitoring, and biological processes.
One way to determine if a substance is an acid or a base is by using indicators. Indicators are substances that change color in the presence of acids or bases. They are often used in titrations, which is a method used to determine the concentration of an acid or base in a solution. The choice of indicator depends on the pH range of the solution being tested.
In acid-base reactions, different types of acids and bases can react with each other to form water and a salt. The reactions can be classified as neutralization reactions, where an acidic and basic solution combine to form a neutral solution. These reactions are often exothermic, meaning they release heat energy.
One common example of acid-base reactions is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH). HCl, a strong acid, reacts with NaOH, a strong base, to form water (H2O) and sodium chloride (NaCl). The balanced equation for this reaction is:
- HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq)
Overall, acid-base reactions and indicators are fundamental concepts in chemistry. They allow scientists to understand and control the behavior of acids and bases, which is crucial for various practical applications.
Common Acids and Bases
Acids and bases are essential substances in chemistry that play important roles in various chemical reactions and processes. They can be found in everyday life, such as in the foods we eat, the beverages we drink, and the cleaning products we use. Understanding common acids and bases is fundamental in understanding their properties and how they interact with other substances.
Acids: Acids are substances that release hydrogen ions (H+) when dissolved in water. They are typically sour-tasting and can cause a burning sensation. Some common acids include:
- Acetic Acid: This is found in vinegar and is responsible for its sour taste.
- Citric Acid: Citrus fruits like lemons and oranges contain citric acid, giving them their tangy taste.
- Sulfuric Acid: Sulfuric acid is a strong acid commonly used in industrial processes such as battery production and steel manufacturing.
Bases: Bases are substances that release hydroxide ions (OH-) when dissolved in water. They are typically bitter-tasting and have a slippery feel. Some common bases include:
- Sodium Hydroxide: Also known as lye, sodium hydroxide is a strong base often used in drain cleaners and soaps.
- Ammonia: Ammonia is a common base found in household cleaners and can also be used as a refrigerant.
- Sodium Bicarbonate: This is commonly known as baking soda and is used in baking and as an antacid to relieve heartburn.
It is important to note that not all acids and bases are safe for consumption or direct contact with skin. Proper handling and precautions should be taken when dealing with these substances to ensure safety. The study of acids and bases is an important area in chemistry that helps scientists understand various chemical reactions and their effects on our daily lives.