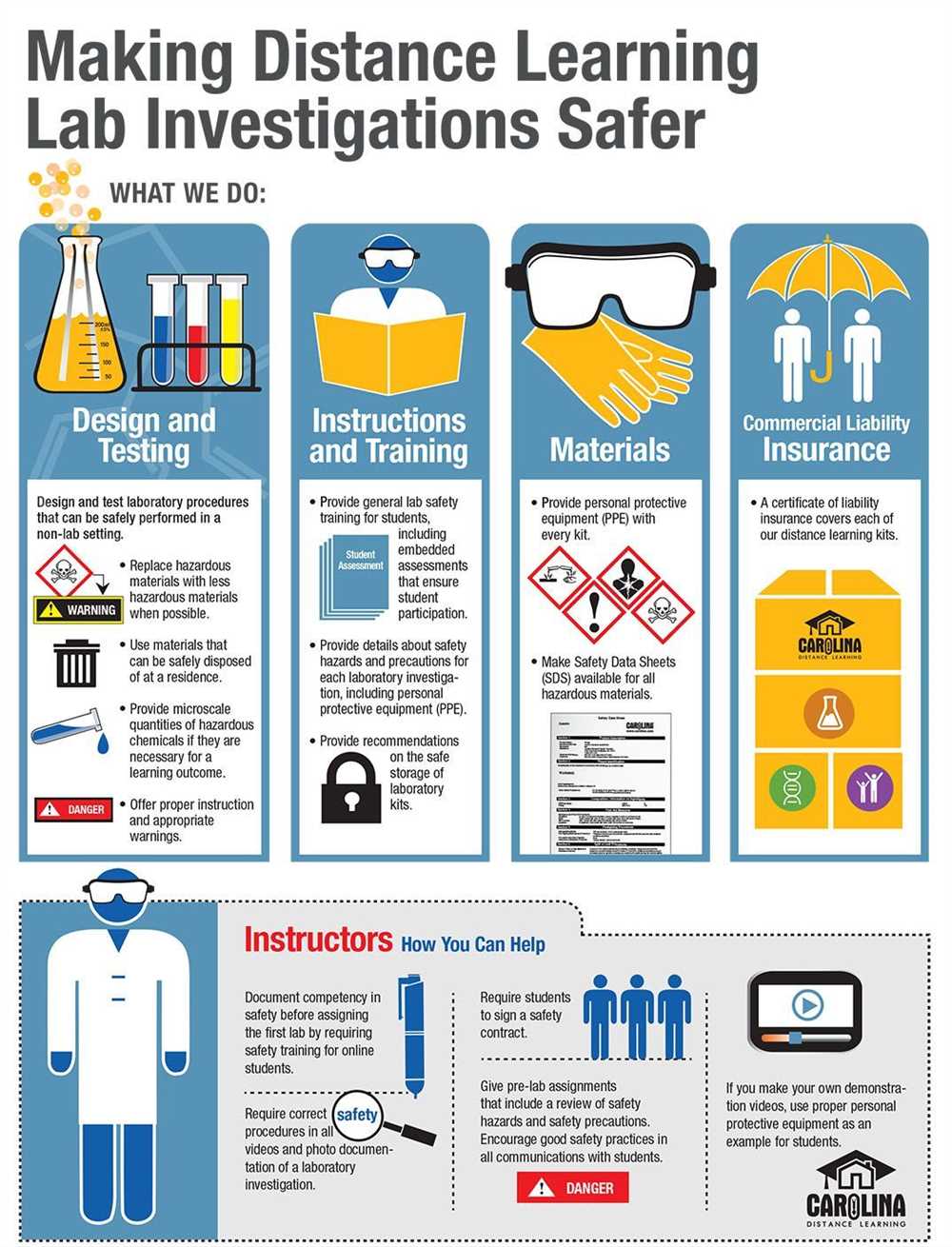
The chemistry behind airbags is a complex and fascinating topic. Airbags are a crucial safety feature in modern vehicles, designed to protect passengers in the event of a collision. The deployment of an airbag relies on a series of chemical reactions that occur within a fraction of a second. Understanding the chemistry involved in airbags is essential for engineers and researchers working to improve their effectiveness and safety.
The main chemical reaction in an airbag deployment is the rapid decomposition of sodium azide (NaN3) into nitrogen gas (N2) and sodium metal (Na). This reaction is triggered when a collision sensor detects a sudden deceleration, causing an electrical current to be sent to the inflator module. The module then initiates the reaction by igniting a small amount of solid fuel, typically a mixture of potassium nitrate (KNO3) and silicon dioxide (SiO2). This ignition releases a high-temperature gas that rapidly decomposes the sodium azide.
Once the sodium azide decomposes, nitrogen gas is produced as the primary inflation gas. Nitrogen is an ideal gas for inflating airbags because it is non-flammable, non-toxic, and inert. The rapid production of nitrogen gas generates a large amount of pressure, causing the airbag to rapidly inflate and fill the desired space. To prevent the airbag from deflating immediately, a small amount of argon or helium gas is also used. These gases have larger atomic masses than nitrogen, providing a longer-lasting inflation and cushioning effect.
In addition to the main chemical reaction, several other chemicals are used in airbags to enhance their effectiveness and safety. For example, a small amount of potassium nitrate may be added to the inflator module to increase the gas production and ensure a rapid inflation. Additionally, talcum powder or cornstarch is often used to coat the inside of the airbag, reducing friction and preventing the fabric from sticking together. This allows the airbag to fully deploy and provide maximum protection to the occupants of the vehicle.
Airbag Lab Chemistry Answers
In the study of airbag lab chemistry, researchers aim to understand the chemical reactions that occur within an airbag during a collision or impact. This knowledge is crucial for designing and developing effective airbag systems that can protect passengers in a vehicle.
Airbag Composition:
An airbag is typically composed of a nylon fabric bag filled with a mixture of chemicals. The most common chemical used in airbag inflation is sodium azide (NaN3). When a collision occurs, the airbag is triggered to inflate rapidly, and this inflation process is driven by a series of chemical reactions.
Chemical Reactions:
The inflation of an airbag involves a two-stage chemical reaction. First, the sodium azide decomposes into sodium metal (Na) and nitrogen gas (N2) according to the following reaction:
NaN3(s) → Na(s) + N2(g)
This decomposition reaction releases a large amount of energy, which in turn rapidly heats up the surrounding gas and causes a rapid expansion of the airbag. However, the nitrogen gas by itself does not provide enough pressure to fully inflate the airbag. Therefore, a second reaction takes place between the nitrogen gas and potassium nitrate (KNO3) to generate additional gas:
N2(g) + 2KNO3(s) → 2KNO2(s) + N2(g) + O2(g)
This reaction produces nitrogen gas, oxygen gas, and potassium nitrite. The released oxygen gas further contributes to the expansion and inflation of the airbag.
Safety Considerations:
While airbags play a crucial role in protecting passengers during accidents, it is important to handle and dispose of the chemicals used in airbags with caution. Sodium azide, for example, is a toxic compound and can be hazardous if handled improperly. It is crucial for manufacturers to follow strict safety protocols in the production and installation of airbags to ensure the safety of both drivers and passengers.
Overall, the study of airbag lab chemistry provides valuable insights into the chemical reactions that occur during airbag inflation. This knowledge allows researchers and engineers to design and optimize airbag systems for maximum safety and protection in the event of a collision or impact.
What is an airbag?
An airbag is a safety device that is designed to provide protection to vehicle occupants in the event of a collision. It is an integral part of the automotive restraint system and works in conjunction with seat belts to reduce the risk of serious injury or death.
The airbag is typically made up of a fabric bag, known as an airbag cushion, an inflation system, and a crash sensor. When a collision occurs, the crash sensor detects the rapid deceleration and sends a signal to the inflation system. The inflation system then rapidly fills the airbag cushion with gas, usually nitrogen or argon, to create a cushioning effect.
Once inflated, the airbag cushion acts as a barrier between the occupant and the hard surfaces of the vehicle, absorbing the impact and reducing the risk of head, neck, and chest injuries. The force of the collision causes the gas to deflate, allowing the occupant to safely exit the vehicle.
Modern vehicles are equipped with multiple airbags strategically placed in different parts of the vehicle, including the front, sides, and sometimes even the rear. This ensures that occupants are protected from various angles and types of collisions.
Components of an Airbag
When it comes to automotive safety features, airbags are one of the most crucial components. An airbag is a supplemental restraint system that works in conjunction with seat belts to protect occupants during a collision. It is designed to inflate rapidly upon impact and then deflate quickly to minimize injury. The key components of an airbag system include the airbag module, sensors, inflator, and the steering wheel or dashboard cover.
Airbag Module
The airbag module is the primary component that houses the airbag and other necessary parts. It consists of a flexible fabric bag made of nylon, which is folded and stored within the steering wheel or dashboard cover. The module also contains an inflator and a chemical propellant that triggers the rapid inflation of the airbag upon impact. The module is typically equipped with a crash sensor that detects a sudden deceleration, signaling the airbag to deploy.
Sensors
Airbags rely on sensors to detect the severity and type of impact. These sensors are strategically placed throughout the vehicle and can include accelerometers, gyroscopes, and impact sensors. The sensors are designed to measure the sudden changes in speed, direction, and deceleration, helping the airbag system determine whether deployment is necessary. The information gathered by the sensors is transmitted to the airbag control module.
Inflator
The inflator is a crucial component responsible for rapidly inflating the airbag upon impact. It consists of a metal or plastic container filled with a chemical propellant, such as sodium azide or tetrazole. When a collision occurs and the crash sensor signals deployment, the inflator ignites the propellant, producing a controlled chemical reaction that rapidly generates a large volume of nitrogen gas. This gas fills the airbag within milliseconds, providing a cushioning effect to reduce the force of impact on the occupants.
Steering Wheel or Dashboard Cover
The steering wheel or dashboard cover houses the airbag module and provides the necessary structural support for deployment. They are typically made of high-strength materials, such as plastic or fiberglass, to withstand the forces involved in a collision. The cover is designed to ensure rapid and controlled deployment of the airbag towards the driver or front passenger, effectively protecting them from hitting the hard surfaces of the steering wheel or dashboard.
In conclusion, the components of an airbag system work together to ensure the safety of vehicle occupants during a collision. The airbag module, sensors, inflator, and steering wheel or dashboard cover all play integral roles in the timely and controlled deployment of the airbag, providing a cushioning effect to reduce potential injuries.
How does an airbag work?

An airbag is a safety device found in most modern vehicles that is designed to protect occupants during a collision. When a vehicle experiences a sudden deceleration, such as in a car crash, the airbag system is triggered to rapidly inflate and provide a cushioning effect for the occupants.
The airbag system consists of several components. The main component is the airbag module, which includes an inflator, a nylon fabric bag, and a sensor. The sensor detects a sudden change in velocity, such as a collision, and sends a signal to the inflator, which contains a chemical mixture called the propellant. When the signal is received, the propellant undergoes a rapid chemical reaction, producing gas and causing the inflator to inflate the airbag.
The airbag itself is made of a strong nylon fabric that can withstand the pressure of the rapidly expanding gas. When the airbag inflates, it fills the space between the occupants and the interior of the vehicle, preventing them from hitting hard surfaces such as the steering wheel, dashboard, or windshield. The force of the impact is absorbed by the airbag, which deflates gradually to allow for a controlled release of gas.
It’s important to note that the airbag system is designed to work in conjunction with seat belts. While the airbag provides an additional layer of protection, seat belts are still the primary safety device in a vehicle. The combination of seat belts and airbags significantly reduces the risk of injury during a collision by helping to distribute the forces of impact and restrain the occupants.
Chemical Reactions in an Airbag
When it comes to airbags, chemical reactions play a crucial role in their function and effectiveness. The main purpose of an airbag is to quickly inflate and provide a cushioning effect during a car accident, preventing serious injuries to the passengers. This rapid inflation is made possible by a series of chemical reactions that occur within the airbag system.
The key component in an airbag is sodium azide (NaN3), a highly reactive compound. When a collision occurs, the airbag control unit sends an electric signal to the inflator module, initiating the chemical reaction. Inside the inflator module, sodium azide rapidly decomposes into its constituent elements: sodium metal (Na) and nitrogen gas (N2). This reaction is highly exothermic, meaning it releases a large amount of heat energy in a short period of time.
- Sodium Azide (NaN3) Decomposition: NaN3 -> Na + N2
As the sodium azide decomposes and the nitrogen gas is produced, the gas rapidly fills the airbag, causing it to inflate within milliseconds. To ensure the safety of the passengers, the airbag is made of a strong and flexible fabric that can withstand the pressure of the expanding gas. The inflation of the airbag creates a cushioning effect, reducing the impact force and protecting the occupants from hitting the hard surfaces of the car.
In addition to sodium azide, an airbag also contains other chemicals such as potassium nitrate, silica, and a mixture of gases like argon and helium. These chemicals are added to modify the characteristics of the gas generated during the inflation process. They help to stabilize the reaction, control the temperature, and prevent the airbag from deploying prematurely or rupturing.
In conclusion, the chemical reactions in an airbag, particularly the rapid decomposition of sodium azide, are essential for the quick inflation and cushioning effect of the airbag during a car accident. These reactions ensure the safety of the passengers by reducing the impact force and preventing severe injuries. The use of other chemicals further enhances the performance and reliability of the airbag system.
Factors Affecting Airbag Inflation
Airbags are an essential safety feature in modern vehicles, designed to protect occupants during collisions by rapidly inflating to cushion the impact. The inflation of airbags is a complex and delicate process, influenced by various factors. Understanding these factors is crucial in ensuring the effectiveness of airbags and the safety of occupants.
1. Crash Severity: The severity of a crash is a significant factor that affects the inflation of airbags. Airbags are designed to deploy only in moderate to severe crashes, where the impact forces exceed a certain threshold. This ensures that airbags are not unnecessarily deployed in minor accidents, which could cause injuries to the occupants.
2. Crash Direction: The direction of the crash also plays a role in airbag inflation. Airbags are typically designed to deploy in frontal crashes, as they are most effective in protecting the head and chest of the occupants from hitting the steering wheel or dashboard. However, some vehicles may have additional side airbags that deploy in side-impact crashes.
3. Occupant Position and Weight: The position and weight of the occupants can affect the timing and force of airbag inflation. Airbags are designed to deploy based on the assumption that the occupants are properly seated and wearing seat belts. If an occupant is out of position, such as leaning forward or not wearing a seat belt, the airbag may not deploy correctly, potentially compromising its effectiveness.
4. Vehicle Speed at Impact: The speed at which the vehicle is traveling at the time of impact can also influence airbag inflation. Higher speeds result in greater forces during a collision, leading to faster and more forceful airbag deployment. On the other hand, lower speeds may result in slower and less forceful inflation. Manufacturers take these variables into account during the design and calibration of airbag systems.
5. Airbag System Design: The design of the airbag system itself is a critical factor affecting inflation. This includes the size and shape of the airbag, the type of propellant used, and the deployment mechanism. Different vehicle manufacturers may use different designs, and advancements in airbag technology continue to improve the effectiveness and safety of these systems.
In conclusion, various factors influence the inflation of airbags, including crash severity, crash direction, occupant position and weight, vehicle speed at impact, and the airbag system design. Understanding and considering these factors is essential in ensuring the optimal performance and safety of airbags during collisions.