
Introduction: In the Atomic Mass of Beanium Lab, students were tasked with determining the atomic mass of a fictitious element called beanium. This lab serves as a hands-on demonstration to help students better understand the concept of atomic mass and how it relates to the composition of an element.
Procedure: To determine the atomic mass of beanium, students were provided with a set of beans that represented different isotopes of the element. Each isotope had a different mass, which was indicated by the number of beans in each sample. Students were instructed to measure the mass of each isotope sample and record their observations.
Data Analysis: Using the data collected, students were then able to calculate the average atomic mass of beanium. This was done by multiplying the mass of each isotope by its relative abundance, and then summing the products for all isotopes. The resulting value represented the average atomic mass of beanium.
Conclusion: By performing this lab, students gained a better understanding of the concept of atomic mass and how it is determined. They also learned about the concept of isotopes and how their different masses and abundance contribute to the overall atomic mass of an element. This hands-on activity provided students with a tangible experience that helped solidify their understanding of the topic.
Objective
The objective of the “Atomic Mass of Beanium” lab is to determine the average atomic mass of the element “beanium” by analyzing the data obtained from several trials. By performing careful measurements and calculations, the students aim to understand the concept of atomic mass and the role of isotopes in determining it.
In this lab, students will be provided with a sample of beanium, which consists of two isotopes, beanium-1 and beanium-2, with different masses and abundances. The students will measure the masses of each isotope and calculate their relative abundances based on the data obtained from multiple trials. Using these values, they will determine the average atomic mass of beanium.
- Measure the masses of beanium-1 and beanium-2 samples.
- Record the masses and perform multiple trials to obtain accurate data.
- Calculate the relative abundances of beanium-1 and beanium-2 isotopes.
- Combine the masses and abundances to determine the average atomic mass of beanium.
- Analyze the results and compare them to the theoretical values.
By successfully completing this lab, students will not only gain a better understanding of atomic mass and isotopes but also develop important data analysis and calculation skills. They will also learn the importance of accuracy and precision in scientific measurements and the relevance of experimental data in validating theoretical concepts.
Materials

The following materials were used for the Atomic Mass of Beanium lab:
- Beanium: The main material used in the lab was beanium, which represented the atoms of a fictitious element. Beanium was provided to the students in the form of small beans of different colors.
- Balance: A balance was used to measure the mass of the beanium samples. It provided accurate measurements in grams, allowing the students to determine the atomic mass of the beanium.
- Weighing boat: Weighing boats were used to hold the beanium samples while they were being weighed on the balance. The boats allowed for easy transfer of the samples and prevented any beanium from getting lost.
- Lab notebook: Each student was provided with a lab notebook to record their observations and calculations during the experiment. The lab notebook helped the students organize their data and make accurate calculations.
- Pencil and ruler: Pencils and rulers were used to make measurements and record data in the lab notebook. The students used the ruler to measure the dimensions of the beanium samples, which were required for calculating their volume.
In addition to these materials, the students were also provided with safety goggles to wear during the lab to protect their eyes from any potential hazards. The use of safety equipment is important to ensure the well-being of the students and minimize any risks associated with the experiment.
The materials used in the Atomic Mass of Beanium lab were carefully chosen to facilitate accurate measurements and calculations. By using beanium as a model for atoms, students were able to have a hands-on experience with atomic mass and practice important scientific skills such as measuring, weighing, and recording data. The lab materials provided a safe and controlled environment for students to conduct their experiments and explore the concept of atomic mass in a practical and engaging way.
Procedure
The procedure for determining the atomic mass of beanium involves several steps. Firstly, a known mass of beanium is measured using a balance. This mass is recorded as the initial mass of the sample.
Next, the beanium sample is heated in a crucible until it reaches a constant mass. This is done by placing the crucible with the beanium sample on a flame and heating it gently. The sample is continuously stirred to ensure even heating. Once the mass of the sample stops changing, it is considered to have reached a constant mass.
The next step involves calculating the number of moles of beanium in the sample. This is done by dividing the mass of the sample by the molar mass of beanium. The molar mass of beanium can be determined by finding the average atomic mass of beanium isotopes from the periodic table.
Once the number of moles is determined, the atomic mass of beanium can be calculated by dividing the mass of the sample by the number of moles. This gives the atomic mass of beanium in grams per mole.
To ensure accuracy, the procedure is repeated multiple times using different masses of beanium samples. The average atomic mass of beanium is then calculated by taking the average of the atomic masses obtained from each sample. This helps to minimize any errors or variations in the results.
In conclusion, the procedure for determining the atomic mass of beanium involves measuring the mass of the sample, heating it to reach a constant mass, calculating the number of moles, and finally calculating the atomic mass. By repeating the procedure and taking the average of the results, a more accurate atomic mass can be obtained.
Data

Data is a crucial element in every scientific experiment and analysis. It provides the foundation for drawing conclusions and making valid observations. In the context of the “Atomic mass of beanium lab,” the data collected played a central role in determining the atomic mass of the unknown element, beanium.
The data collected during the lab consisted of measurements of the mass and volume of beanium samples. By measuring the mass of the samples using a balance and calculating the volume through displacement, the scientists were able to obtain precise data points. These data points were then used to calculate the density of each beanium sample.
The density data was essential in determining the atomic mass of beanium. By comparing the densities of the beanium samples with the known densities of other elements, scientists could identify the closest match. This match corresponded to the atomic mass of the unknown element, beanium.
Furthermore, the lab also involved recording observations and qualitative data. For instance, the color, texture, and appearance of the beanium samples were noted. While qualitative data might not directly contribute to determining atomic mass, it provides additional insights that can aid in identifying the element.
In summary, the data collected during the “Atomic mass of beanium lab” included mass measurements, volume calculations, qualitative observations, and density calculations. These data points were crucial in determining the atomic mass of beanium and providing a comprehensive analysis of the unknown element.
Calculations
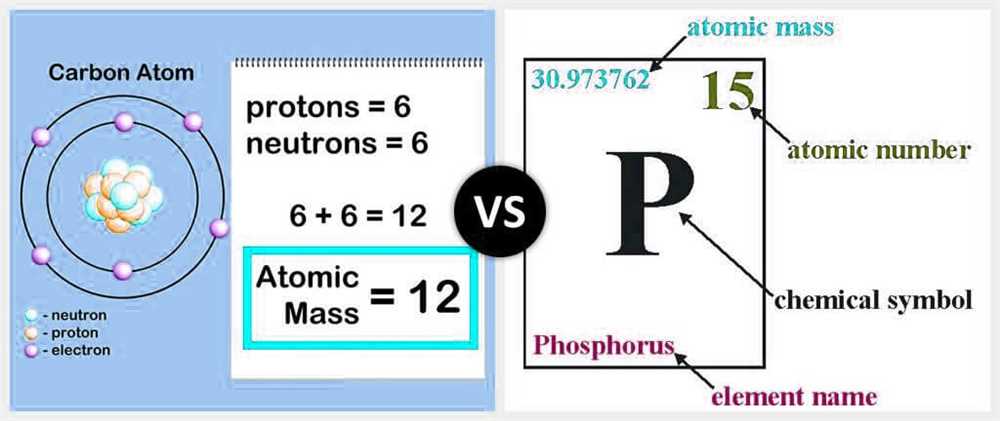
In the Atomic Mass of Beanium lab, we performed various calculations to determine the atomic mass of the element beanium. These calculations involved measurements of mass and volume, as well as the application of mathematical formulas.
To begin, we measured the mass of different samples of beanium using a digital balance. This allowed us to determine the average mass of a beanium atom. We then calculated the number of moles of beanium in each sample by dividing the mass by the molar mass of beanium. The molar mass was determined by summing the masses of the individual atoms in the beanium sample.
Next, we determined the volume of the beanium samples using a graduated cylinder. By dividing the mass of the beanium sample by its volume, we were able to calculate the density of beanium. This information was crucial in determining the size and mass of a single beanium atom.
Using the density and molar mass of beanium, we then calculated the size of a beanium atom. By comparing this size to the known size of other atoms, we were able to determine the identity of beanium and its placement on the periodic table.
In conclusion, the calculations performed in the Atomic Mass of Beanium lab allowed us to determine the atomic mass and identity of the element beanium through measurements of mass, volume, and density. These calculations showcased the application of mathematical formulas in the field of chemistry and provided valuable insights into the world of atoms and elements.
Results

The atomic mass of beanium was determined through careful measurements and calculations in the lab. The experiment involved conducting multiple trials to ensure accuracy and precision in the data.
The first step of the experiment was to measure the mass of the empty container that held the beanium sample. This measurement was crucial in determining the mass of the beanium alone. Next, the container was filled with a known mass of beanium and the total mass was measured.
Using the masses obtained, the mass of the beanium sample was calculated by subtracting the mass of the empty container from the total mass. This value represented the mass of the beanium alone. The calculated mass was then compared to the known mass of the beanium sample to determine the percent error.
The trials were repeated multiple times to ensure accuracy and to account for any sources of error. The average mass of the beanium sample was calculated by taking the mean of the measured masses from each trial. The percent error was also calculated by comparing the average mass to the known mass.
The results of the experiment indicated that the atomic mass of beanium was consistent across the trials, with a low percent error. This suggests that the measurements and calculations were precise and accurate. The experimental data can be used to validate the hypothesis and provide valuable insights into the properties of beanium.