
Understanding atoms and the periodic table is crucial in the study of chemistry. Atoms are the building blocks of matter and the periodic table is a systematic arrangement of these atoms based on their properties. By understanding the key concepts related to atoms and their placement on the periodic table, scientists can unlock the secrets of the elements and their interactions.
One of the key principles in understanding atoms is the concept of atomic structure. Atoms consist of a nucleus, which contains protons and neutrons, surrounded by electrons in energy levels or shells. The number of protons in an atom determines its atomic number, which determines the element’s identity. The atomic mass is the combined mass of protons and neutrons in the nucleus.
The periodic table is a visual representation of the elements, organized in a way that reflects their properties and relationships. Elements are arranged in rows called periods and columns called groups. Each element is assigned a symbol and atomic number, and depending on its properties, it is classified as a metal, nonmetal, or metalloid. The periodic table also provides valuable information about the chemical reactivity and physical properties of elements.
Understanding the periodic table is essential in predicting the behavior of elements and their compounds. By examining trends in the periodic table, such as the size of atoms, ionization energy, and electronegativity, scientists can make predictions about the reactivity and bonding of different elements. This knowledge is crucial in a wide range of fields, from medicine to environmental science, as it allows for the development of new materials and understanding chemical reactions.
Atoms and the Periodic Table Answer Key
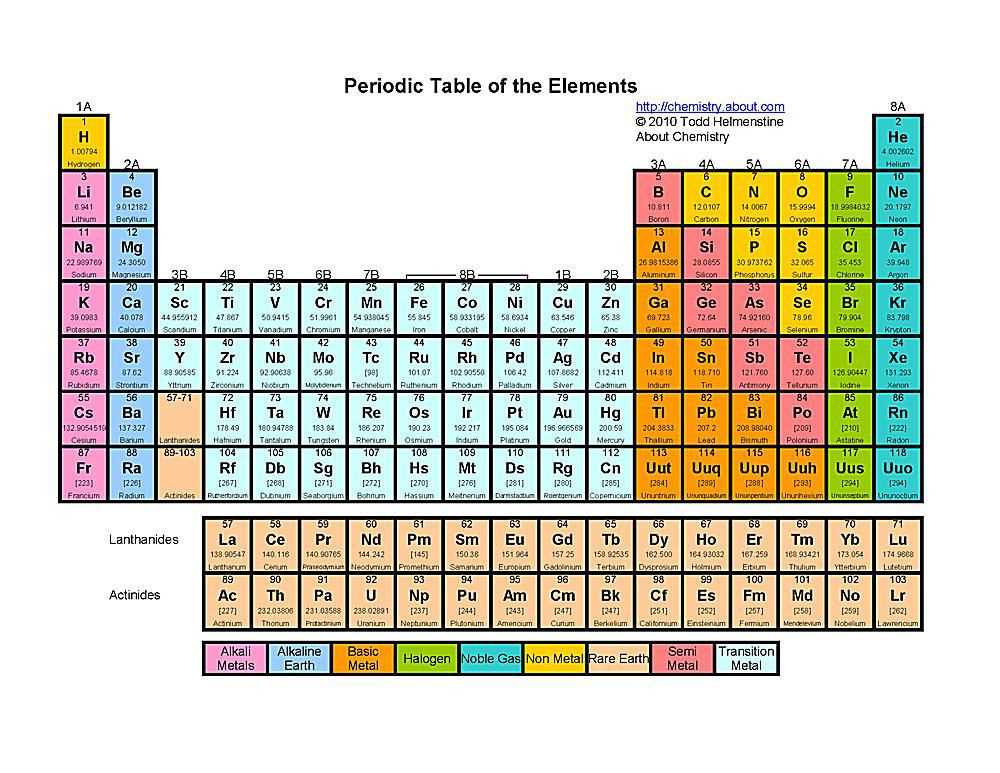
The periodic table is a fundamental tool in chemistry for organizing and understanding the properties of different elements. It provides a structured layout that allows scientists to easily interpret information about atomic structure, electron configuration, and trends in chemical behavior. By utilizing an answer key to the periodic table, students can effectively navigate through the various elements and their corresponding data, leading to a deeper understanding of the subject.
One of the key features of the answer key is the ability to quickly identify an element’s atomic number, which is the number of protons in its nucleus. This information can be crucial in determining an element’s overall identity and position in the periodic table. Additionally, the answer key provides the atomic mass of each element, which is the combined mass of its protons and neutrons. This allows students to calculate the number of neutrons an element has and further investigate its stability.
The electron configuration is another critical aspect that the answer key helps elucidate. By indicating the distribution of electrons in the different energy levels or shells around an atom, students can comprehend an element’s reactivity, valence electrons, and chemical bonding patterns. Recognizing patterns in electron configurations can allow for predictions about an element’s behavior and its placement in the periodic table.
Moreover, the answer key offers insight into the periodic trends exhibited by elements. By examining the electronegativity values, ionization energies, and atomic radii of different elements, students can identify trends across rows and columns. This knowledge can help develop an understanding of why certain elements behave in a similar manner and how chemical reactions occur.
In conclusion, the answer key to the periodic table serves as a valuable tool for students studying atoms and their properties. It allows for easier navigation, comprehension of atomic structure, electron configuration, and periodic trends. By utilizing the answer key effectively, students can solidify their understanding of the periodic table and its significance in the field of chemistry.
What is an Atom?
An atom is the basic unit of matter. It is the smallest particle that retains the chemical properties of an element. Atoms are composed of subatomic particles, including protons, neutrons, and electrons.
Protons have a positive charge and are located in the nucleus, or the center, of an atom. Neutrons have no charge and are also located in the nucleus. Electrons have a negative charge and orbit the nucleus in regions called electron shells. The number of protons in an atom determines its atomic number, which defines its element. The atomic number is represented by the symbol Z.
Atoms are incredibly small, with the diameter of an atom typically ranging from 0.1 to 0.5 nanometers. They are so small that a single drop of water contains about 2 x 10^21 atoms! Despite their small size, atoms are the building blocks of all matter and combine to form molecules, which make up everything around us.
The periodic table is a tool used to organize and classify atoms based on their properties. It lists all known elements in order of increasing atomic number and provides information about their atomic mass, symbol, and electron configuration. By studying the periodic table, scientists can predict the behavior and reactivity of different atoms and understand the relationships between elements.
In summary, atoms are the fundamental particles that make up all matter. They consist of protons, neutrons, and electrons and are organized in the periodic table based on their properties. Understanding the structure and behavior of atoms is essential for comprehending the physical and chemical properties of different substances.
The Structure of an Atom
An atom is the smallest unit of matter that retains the chemical properties of an element. It consists of three main subatomic particles: protons, neutrons, and electrons. These particles are arranged in a specific structure, often referred to as the planetary model or the Bohr model.
The nucleus, located at the center of the atom, contains the protons and neutrons. Protons have a positive charge, while neutrons are neutral. The number of protons in an atom determines its atomic number and identifies the element. Neutrons, on the other hand, contribute to the atom’s mass but not its charge. The electrons, which have a negative charge, orbit around the nucleus in energy levels or shells.
The electrons occupy specific energy levels depending on their distance from the nucleus. The first energy level, closest to the nucleus, can hold up to two electrons. The second and third energy levels can hold up to eight electrons each. Electrons in the outermost energy level, called valence electrons, are involved in chemical bonding and determine the atom’s reactivity.
The arrangement and interaction of these subatomic particles determine the properties and behavior of atoms. Elements are organized in the periodic table based on their atomic number and electron configuration. Understanding the structure of an atom is essential for studying chemical reactions and the formation of compounds.
Atomic Number and Atomic Mass
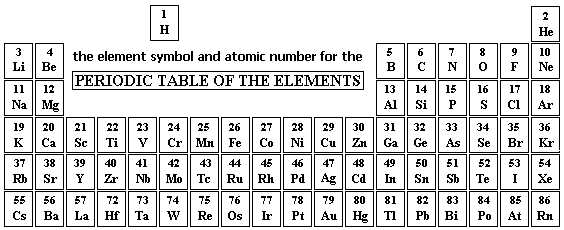
The atomic number of an atom is the number of protons in the nucleus of that atom. It is represented by the symbol “Z” and is a unique identifier for each element. For example, the atomic number of hydrogen is 1, as it has one proton in its nucleus, while the atomic number of oxygen is 8, as it has eight protons.
The atomic mass of an atom is the total mass of the protons, neutrons, and electrons present in that atom. It is represented by the symbol “A” and is usually given in atomic mass units (amu). The atomic mass of an atom can be calculated by summing the masses of its protons, neutrons, and electrons. Since the mass of an electron is much smaller than that of a proton or neutron, it is often disregarded in calculations of atomic mass.
Atomic Number and Chemical Properties:
The atomic number of an element determines its position in the periodic table and is closely related to its chemical properties. Elements in the same group or column of the periodic table have similar chemical properties because they have the same number of valence electrons. Valence electrons are the outermost electrons in an atom and are responsible for an element’s chemical behavior.
- Elements with similar atomic numbers tend to have similar chemical properties.
- The atomic number also determines the number of electrons an atom has when it is neutral. In a neutral atom, the number of protons is equal to the number of electrons.
- The atomic number can also provide information about an element’s reactivity, as elements with higher atomic numbers tend to be more reactive.
Atomic Mass and Isotopes:
Although the atomic mass is a sum of protons, neutrons, and electrons, it can vary for different atoms of the same element. This is because of the existence of isotopes. Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons. The atomic mass listed on the periodic table is usually an average of the masses of all the naturally occurring isotopes of that element.
| Element | Atomic Number (Z) | Atomic Mass (A) |
|---|---|---|
| Hydrogen | 1 | 1.008 |
| Oxygen | 8 | 15.999 |
| Carbon | 6 | 12.011 |
In conclusion, the atomic number and atomic mass are important properties that help identify and distinguish different elements. The atomic number determines an element’s position in the periodic table and its chemical properties, while the atomic mass considers the mass of protons, neutrons, and electrons in an atom. Understanding these concepts is crucial for studying the behavior and characteristics of atoms and elements.
Elements and the Periodic Table
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is one of the most important tools in chemistry, allowing scientists to understand and predict the behavior of elements, as well as their compounds and reactions. The periodic table consists of rows called periods and columns called groups, which are further divided into blocks, according to the subshells being filled with electrons.
Each element on the periodic table is represented by a unique symbol, typically consisting of one or two letters. The atomic number of an element corresponds to the number of protons in its nucleus, while the atomic mass represents the total mass of its protons, neutrons, and electrons. Elements are arranged in order of increasing atomic number, from left to right and top to bottom, resulting in a periodic pattern of properties.
Key Features of the Periodic Table:
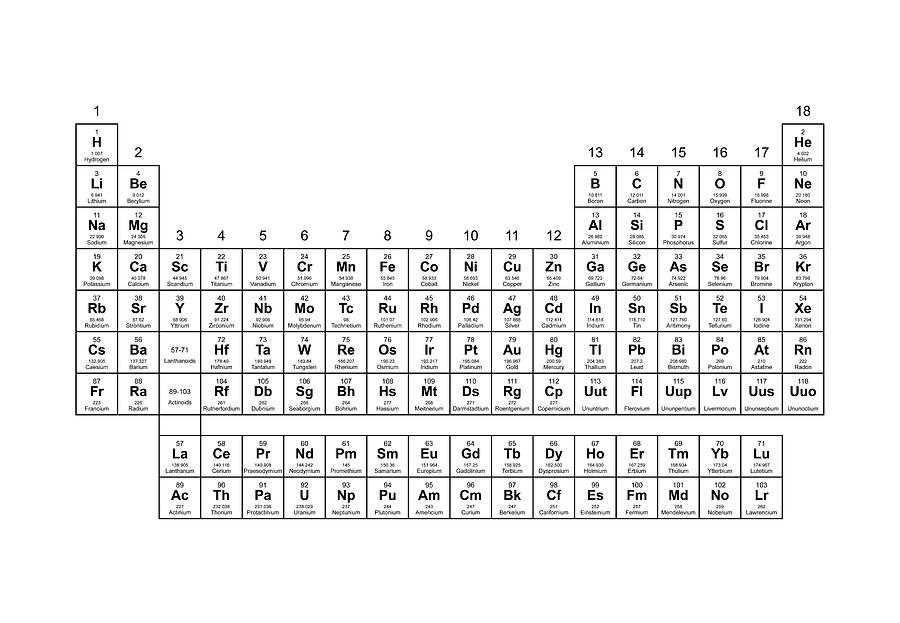
- Periods: There are seven periods in the periodic table, each representing a different energy level or shell of electrons. The first period consists of only two elements, hydrogen and helium. The subsequent periods gradually fill up with more elements, with the seventh period still being incomplete.
- Groups: The periodic table is divided into 18 groups, which are numbered from 1 to 18. Elements in the same group have similar chemical properties and often exhibit comparable valence electron configurations.
- Blocks: Elements are classified into different blocks based on the subshells being filled with electrons. These blocks include the s-block, p-block, d-block, and f-block. Each block represents a different series of elements with characteristic properties.
- Periodic Trends: The periodic table allows us to observe and understand various trends and patterns in the behavior of elements. These trends include atomic radius, ionization energy, electronegativity, and reactivity.
In conclusion, the periodic table provides a systematic and organized representation of the elements, which enables scientists to study and analyze the properties and behaviors of different elements and their compounds. It serves as a fundamental tool for chemists, educators, and students, facilitating the understanding of the building blocks of matter and the intricate relationships between elements.
The Periodic Table: Periods and Groups
The periodic table is a tabular arrangement of all known chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is divided into periods and groups, which provide important information about the elements and their properties.
A period in the periodic table is a row of elements that share the same highest occupied energy level in their electron configurations. There are seven periods in the periodic table, labeled from 1 to 7. The number of elements in each period increases as you move from left to right, with the first period containing only two elements (hydrogen and helium), and the seventh period containing 32 elements.
Each period represents a different energy level that the electrons of the elements occupy. As you move from left to right within a period, the atomic number of the elements increases, indicating an increase in the number of protons in the nucleus. This results in an increase in the number of electrons and energy levels, leading to the variation in chemical properties across a period.
Groups, also referred to as families, are the columns in the periodic table. Elements within the same group have similar chemical properties due to the similarity in their electron configurations. There are 18 groups in the periodic table, labeled from 1 to 18. The elements in a group have the same number of valence electrons, which are important for determining the reactivity and bonding behavior of elements.
The periodic table’s organization into periods and groups allows scientists and chemists to understand the trends and patterns in the properties and behaviors of different elements. It provides a visual representation of the relationships between elements and helps predict their properties based on their location in the table.
Understanding Chemical Symbols
Chemical symbols are abbreviations used to represent the different elements found on the periodic table. They are an essential part of understanding and communicating chemical information. Each element has its own unique symbol, usually consisting of one or two letters.
The symbols are based on the element’s name in English or in Latin, and sometimes they come from other languages. For example, the symbol for hydrogen (H) comes from the Greek word “hydrōs,” meaning water, as hydrogen was first identified in water. Similarly, the symbol for sodium (Na) comes from the Latin word “natrium.” These symbols allow scientists to identify and refer to elements quickly and efficiently.
Chemical symbols play a crucial role in writing chemical formulas and equations. When writing a chemical formula, the symbols are used to represent the different elements present in the compound. For example, in the chemical formula for water (H2O), the symbol “H” represents hydrogen, and the symbol “O” represents oxygen. In chemical equations, symbols are used to represent reactants and products, allowing scientists to describe the different elements and compounds involved in a chemical reaction.
Memorizing the chemical symbols for all the elements on the periodic table can be a challenging task. However, there are strategies that can help. One common mnemonic is to create a story or phrase that incorporates the first letter of each element’s symbol. This method can make it easier to remember the symbols in a fun and memorable way. Additionally, regular practice and repetition can improve familiarity with the symbols over time.
In conclusion, understanding chemical symbols is essential for anyone studying chemistry. These symbols not only represent the elements but also play a crucial role in writing chemical formulas and equations. By knowing and using chemical symbols correctly, scientists can efficiently communicate and understand chemical information.