
ATP (adenosine triphosphate) is a molecule that serves as a crucial source of energy within cells. It is often referred to as the “energy currency” of the cell because it provides the energy needed for various metabolic processes. In order to understand how ATP functions as an energy carrier, it is important to explore the structure and function of this molecule.
The structure of ATP consists of three main components: a ribose sugar, a nitrogenous base (adenine), and three phosphate groups. The phosphate groups are linked together by high-energy bonds, which store a significant amount of potential energy. When one of these phosphate groups is removed, ATP is converted into ADP (adenosine diphosphate) and inorganic phosphate (Pi), releasing energy in the process.
ATP acts as a versatile energy carrier in the cell, providing energy for various processes such as muscle contraction, active transport, and chemical synthesis. It accomplishes this by undergoing a cycle of synthesis and hydrolysis. During synthesis, energy from cellular respiration or photosynthesis is used to convert ADP and Pi back into ATP. This process, known as phosphorylation, requires the input of energy. The formed ATP can then be used as an energy source during hydrolysis reactions, where it is converted back into ADP and Pi, releasing energy for cellular processes.
Overall, ATP plays a crucial role as a free energy carrier in cells. Its structure and ability to undergo phosphorylation and hydrolysis allow it to efficiently store and release energy as needed. This energy currency is essential for the proper functioning of cells and is involved in a wide range of metabolic activities. Understanding the importance and mechanisms of ATP can provide valuable insights into cellular energy metabolism and the overall functioning of living organisms.
Atp Free Energy Carrier Pogil Answers
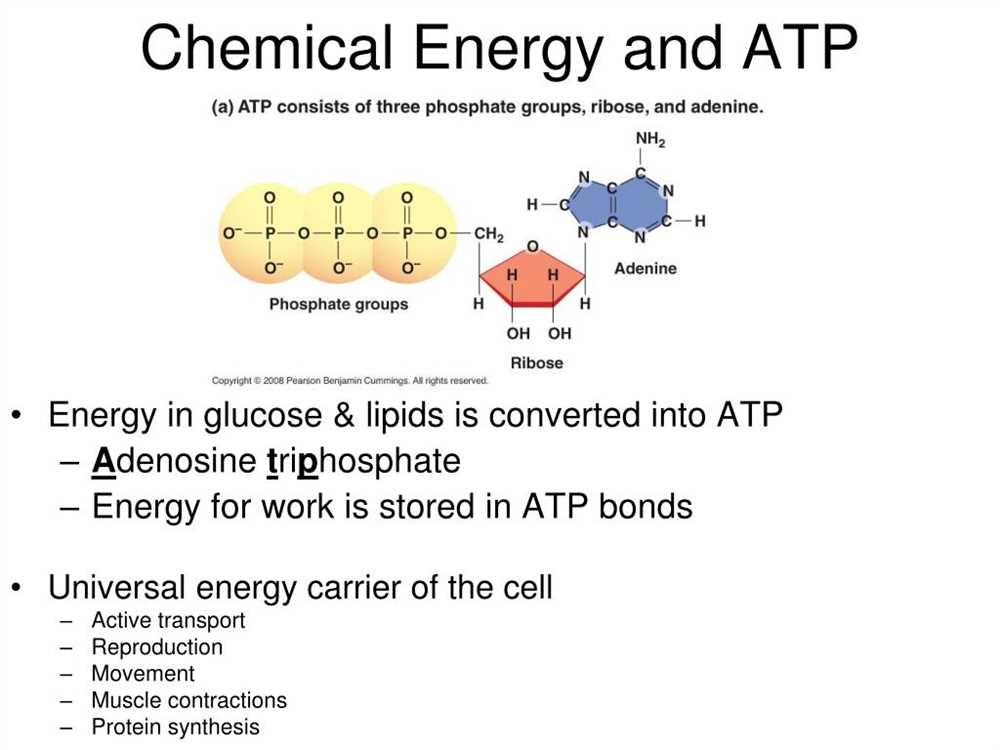
ATP, or adenosine triphosphate, is often referred to as the “energy currency” of the cell. It is a molecule that stores and transfers energy within cells, allowing them to perform various functions. In the context of POGIL (Process Oriented Guided Inquiry Learning), students are often presented with a set of questions or scenarios related to ATP and its role as a free energy carrier. The answers to these questions can help students deepen their understanding of ATP and its importance in cellular processes.
One of the questions that students may encounter in an ATP POGIL activity is: “What are the three phosphate groups in ATP called?” The correct answer to this question is: alpha, beta, and gamma phosphates. These phosphate groups are attached to the adenosine molecule and can be hydrolyzed to release energy. Another question that students may come across is: “How is ATP synthesized in cells?” The correct answer to this question is: through the process of cellular respiration, specifically during the electron transport chain and oxidative phosphorylation.
Another important concept that students may explore in an ATP POGIL activity is the role of ATP in cellular processes. One question that may be asked is: “Why is ATP necessary for active transport?” The correct answer to this question is: ATP provides the energy needed to move molecules against their concentration gradient, a process that requires energy input. ATP is also essential for muscle contraction, DNA replication, and protein synthesis. It serves as a source of energy for various cellular processes and is continuously regenerated through cellular respiration.
In conclusion, an ATP POGIL activity provides students with the opportunity to explore the role of ATP as a free energy carrier in cells. By answering questions related to ATP synthesis, structure, and function, students can deepen their understanding of this crucial molecule and its importance in cellular processes.
What is ATP?
ATP stands for adenosine triphosphate. It is a molecule that serves as the main energy carrier in living organisms. ATP is composed of three main components: the adenine base, ribose sugar, and a chain of three phosphate groups.
ATP acts as a universal currency for energy in cells, transferring energy from where it is generated to where it is needed. It is often referred to as the “energy currency” of the cell. The high-energy phosphate bonds in ATP store the energy that is needed for cellular processes.
The hydrolysis of ATP, in which a phosphate group is removed, releases energy that can be used to power cellular processes such as muscle contraction, active transport, and chemical reactions. When ATP is hydrolyzed, it forms adenosine diphosphate (ADP) and an inorganic phosphate (Pi). This process can be reversed through the addition of a phosphate group to ADP, which reforms ATP.
In summary, ATP is a crucial molecule that stores and transfers energy in cells. It plays a fundamental role in various cellular processes and is essential for the functioning of living organisms.
Structure of ATP

ATP, or adenosine triphosphate, is a molecule that serves as the primary energy currency of cells. The structure of ATP consists of three main components: an adenine molecule, a ribose sugar molecule, and three phosphate groups.
The adenine molecule is a nitrogenous base derived from purine. It is connected to the ribose sugar molecule through a glycosidic bond. The ribose sugar molecule, a pentose sugar, provides the backbone for the entire ATP molecule. It is connected to the phosphate groups through phosphoester bonds.
The three phosphate groups are the key feature of ATP. They are attached to the ribose sugar molecule in a chain-like structure. The first and second phosphate groups are attached through phosphoanhydride bonds, which are high-energy bonds. The third phosphate group is attached through a phosphoester bond.
The high-energy bonds between the phosphate groups store a significant amount of chemical potential energy. This energy is released when ATP is hydrolyzed, breaking one or two of the phosphoanhydride bonds and forming adenosine diphosphate (ADP) or adenosine monophosphate (AMP), respectively.
In summary, the structure of ATP consists of an adenine molecule, a ribose sugar molecule, and three phosphate groups. The high-energy bonds between the phosphate groups store energy that can be released for cellular processes. ATP plays a crucial role as a free energy carrier in various biological reactions.
Formation of ATP
ATP, or adenosine triphosphate, is a molecule that serves as the primary form of energy currency in cells. It is formed through a process called cellular respiration, which occurs in the mitochondria of cells. During cellular respiration, molecules such as glucose are broken down in a series of reactions, ultimately producing ATP.
ATP Synthase: One of the key enzymes involved in the formation of ATP is ATP synthase. This enzyme is located in the inner mitochondrial membrane and utilizes the energy released from the flow of protons (H+) across the membrane to drive the synthesis of ATP. ATP synthase acts like a turbine, converting this energy into the formation of chemical bonds in ATP.
Energy Storage: ATP is a high-energy molecule due to the arrangement of its phosphate groups. When ATP is formed, it contains three phosphate groups. The last two phosphate groups are connected by high-energy bonds. When one phosphate group is removed from ATP, through a process called hydrolysis, the bond is broken and energy is released. This energy can then be used to power various cellular processes.
Overall, the formation of ATP is a crucial process in cellular respiration. It allows cells to efficiently store and utilize energy for a variety of functions, including muscle contraction, active transport, and DNA synthesis. Without ATP, cells would not be able to perform essential tasks and would ultimately cease to function.
Role of ATP in Cells
Adenosine triphosphate (ATP) is a crucial molecule in cells that functions as an energy carrier. ATP is composed of adenosine, a nitrogenous base, and three phosphate groups. It possesses a high-energy bond between the second and third phosphate groups, which can be broken to release energy. This energy release is used by cells to perform various metabolic processes and provide energy for cellular activities.
Energy Currency: ATP is often referred to as the “energy currency” of the cell because it stores and transfers energy in a form that is readily available for use. When ATP is hydrolyzed, the bond between the second and third phosphate groups breaks, releasing one phosphate group and forming adenosine diphosphate (ADP) and inorganic phosphate (Pi). This hydrolysis reaction releases a significant amount of energy that can be used by cells to drive other chemical reactions.
Cellular Work: ATP is involved in various biological processes and provides the necessary energy for cellular work. It powers active transport processes, such as the movement of ions across cell membranes, against their concentration gradients. ATP also drives muscle contraction, enabling movement in organisms. Furthermore, ATP is essential in biochemical reactions, such as the synthesis of macromolecules like proteins and nucleic acids.
- Synthesis: ATP is used as an energy source for the synthesis of macromolecules. The energy released from ATP hydrolysis is used to drive endergonic reactions, which require an input of energy. For example, ATP provides the energy needed for the formation of peptide bonds during protein synthesis.
- Signal Transduction: ATP is also involved in cell signaling processes. It acts as a signaling molecule and is released from cells in response to extracellular stimuli. The released ATP can bind to specific receptors on nearby cells or act as a substrate for enzymes, initiating signaling cascades and triggering cellular responses.
- Enzymatic Reactions: ATP often serves as a coenzyme or cofactor for enzymes involved in metabolic reactions. It can donate phosphate groups to molecules, activating them for further reactions. For example, ATP donates a phosphate group to glucose during glycolysis, facilitating its breakdown and subsequent energy release.
In summary, ATP plays a critical role in cells as an energy carrier and currency. It provides the necessary energy for cellular work, drives various biochemical reactions, and participates in cell signaling and enzymatic processes. Without ATP, cellular activities and essential metabolic processes would not be able to occur efficiently.
ATP as an Energy Currency

Adenosine triphosphate (ATP) is often referred to as the energy currency of cells. It serves as a universal carrier of chemical energy within living organisms. ATP is composed of three phosphate groups, a ribose sugar, and adenine. The high-energy bonds between the phosphate groups make ATP an ideal molecule for storing and releasing energy.
ATP acts as a source of energy for various cellular processes, such as muscle contraction, active transport, and synthesis of macromolecules. When ATP is hydrolyzed, a phosphate group is removed, resulting in the formation of adenosine diphosphate (ADP) and inorganic phosphate (Pi). This hydrolysis reaction releases energy that can be utilized by cells.
ATP regeneration is crucial for sustaining cellular energy levels. The process of regenerating ATP from ADP and Pi occurs through cellular respiration, specifically oxidative phosphorylation and substrate-level phosphorylation. During these processes, energy derived from the breakdown of nutrients is used to synthesize ATP from ADP and Pi, replenishing the cellular energy pool.
In summary, ATP plays a vital role as an energy currency in cells. Through hydrolysis and regeneration, it allows for the continuous supply of energy required for various cellular functions. Without ATP, cells would not have a readily available source of energy, and essential biological processes would not be able to occur efficiently.
How Does ATP Release Energy?

ATP, or adenosine triphosphate, is often referred to as the “energy currency” of the cell. It serves as a universal molecule for storing and transferring energy within living organisms. The release of energy from ATP occurs through a process called hydrolysis, where ATP is broken down into ADP (adenosine diphosphate) and inorganic phosphate (Pi).
Hydrolysis of ATP is catalyzed by the enzyme ATPase, which transfers a water molecule to the ATP molecule, causing it to split into ADP and Pi. This hydrolysis reaction releases energy in the form of a high-energy phosphate bond. The released energy can be used by cells to drive various metabolic processes, such as muscle contractions, active transport of ions across cell membranes, and synthesis of macromolecules.
ATP hydrolysis is a highly exergonic reaction, meaning it releases a large amount of free energy. This is because the phosphate group in ATP carries negative charges that are repelled by each other, making the bond between the phosphate groups unstable. Therefore, when the phosphate bond is broken during hydrolysis, a more stable state is achieved, resulting in the release of energy.
The energy released from ATP hydrolysis is used to power cellular work, such as mechanical work (such as muscle movement), chemical work (such as synthesis of biomolecules), and transport work (such as pumping ions across cell membranes). After ATP is hydrolyzed and its energy is released, ADP and Pi can be regenerated back into ATP through cellular respiration or other energy-replenishing processes, ensuring a continuous supply of energy for cellular activities.
ATP Hydrolysis Reaction
The ATP hydrolysis reaction plays a crucial role in providing energy for cellular processes. ATP, or adenosine triphosphate, is considered the energy currency of the cell. It is a nucleotide that consists of a sugar (ribose), a nitrogenous base (adenine), and three phosphate groups. The hydrolysis of ATP involves the breaking of the high-energy bond between the last two phosphate groups, resulting in the release of one inorganic phosphate (Pi) molecule and the formation of adenosine diphosphate (ADP).
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. This release of energy is utilized by cells to perform various cellular functions such as active transport, muscle contraction, and synthesis of macromolecules. The hydrolysis reaction is catalyzed by enzymes called ATPases, which facilitate the breaking of the phosphate bond. The energy released during ATP hydrolysis is primarily used to drive endergonic reactions, which require the input of energy. ADP and Pi are then recycled back into ATP through the process of cellular respiration.
The ATP hydrolysis reaction is highly efficient, allowing cells to quickly generate energy when needed. The energy released during hydrolysis is utilized to perform mechanical work, such as the movement of organelles within cells, and to drive chemical reactions by providing the necessary activation energy. Additionally, the hydrolysis of ATP is reversible, with ATP synthesis occurring through the process of phosphorylation. This allows cells to store and utilize energy as needed, depending on the metabolic state and energy demands of the organism.
In summary, the ATP hydrolysis reaction is a key process in cellular energy metabolism. It releases energy that is used to power various cellular functions, and the resulting ADP and Pi can be recycled back into ATP. This continuous cycle of ATP hydrolysis and synthesis allows cells to efficiently utilize and store energy, enabling them to carry out essential biological processes.