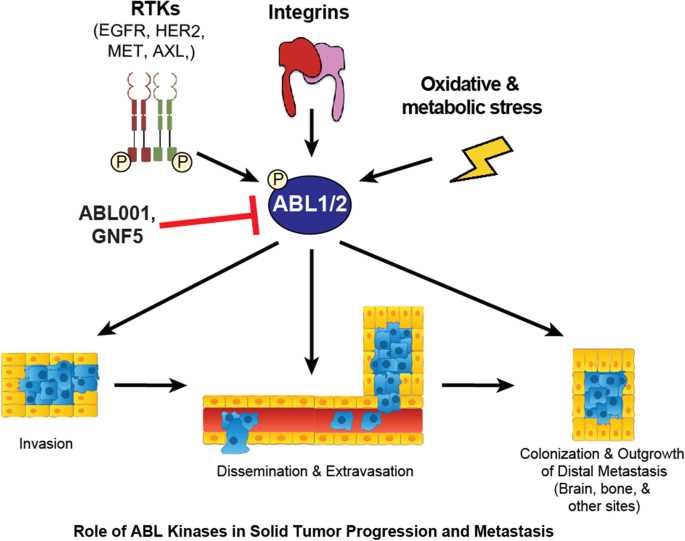
Bcr-Abl is a cancer protein that plays a critical role in the development and progression of Chronic Myeloid Leukemia (CML). Understanding the structure and function of Bcr-Abl is essential for developing targeted therapies to treat this disease and improve patient outcomes.
The Bcr-Abl protein is created as a result of a genetic mutation that fuses the Bcr and Abl genes together. This fusion protein has a unique structure and function that contributes to the development of CML. The Bcr part of the protein acts as a regulatory domain, while the Abl part is a tyrosine kinase domain. This tyrosine kinase activity is crucial for the signaling pathways that promote cell growth and survival.
The abnormal activation of Bcr-Abl protein leads to the uncontrolled proliferation of white blood cells, which is a characteristic feature of CML. Bcr-Abl also inhibits apoptosis, or programmed cell death, allowing cancer cells to survive and accumulate over time. Therefore, targeting Bcr-Abl and blocking its activity is a key strategy for treating CML.
Several drugs have been developed to specifically target Bcr-Abl and inhibit its tyrosine kinase activity. These drugs, such as imatinib, nilotinib, and dasatinib, are known as tyrosine kinase inhibitors (TKIs) and have revolutionized the treatment of CML. They bind to the ATP binding site of the Abl kinase domain, preventing the transfer of phosphate groups to target proteins and disrupting the signaling pathways that drive cancer growth.
In conclusion, understanding the structure and function of the Bcr-Abl protein is crucial for developing effective treatments for CML. Targeted therapies that inhibit the abnormal activity of Bcr-Abl have significantly improved patient outcomes and survival rates. Ongoing research on Bcr-Abl and its role in other cancers may lead to the development of novel therapies for a variety of malignancies.
Bcr-Abl Cancer Protein Structure and Function Answer Key
The Bcr-Abl protein is a fusion protein that arises from a translocation event between the Bcr and Abl genes. This translocation event creates an abnormal fusion gene, which produces the Bcr-Abl protein. The Bcr-Abl protein is a constitutively active tyrosine kinase that drives the development of chronic myeloid leukemia (CML).
The structure of the Bcr-Abl protein is composed of several functional domains. The Abl domain is the catalytic domain of the protein and is responsible for its tyrosine kinase activity. The Bcr domain, on the other hand, acts as a regulatory domain and helps to stabilize the protein. Additionally, the Bcr domain also contains a region called the coiled-coil domain, which plays a role in dimerization of the Bcr-Abl protein.
The function of the Bcr-Abl protein is closely linked to its structure. The constitutively active tyrosine kinase activity of the Abl domain leads to the unregulated phosphorylation of various downstream signaling molecules, resulting in the dysregulation of cell growth and survival pathways. This abnormal signaling cascade ultimately leads to the development of CML.
Targeting the Bcr-Abl protein has been a successful therapeutic strategy for the treatment of CML. The development of tyrosine kinase inhibitors (TKIs), such as imatinib, dasatinib, and nilotinib, has revolutionized the management of CML. These TKIs specifically bind to the ATP-binding site of the Abl domain, inhibiting its kinase activity and blocking the downstream signaling cascade.
- In conclusion, the Bcr-Abl protein is a fusion protein that arises from a translocation event between the Bcr and Abl genes.
- The structure of the Bcr-Abl protein consists of the Abl domain, which is responsible for its tyrosine kinase activity, and the Bcr domain, which acts as a regulatory domain.
- The constitutively active tyrosine kinase activity of the Bcr-Abl protein leads to the dysregulation of cell growth and survival pathways, resulting in the development of CML.
- Treatment options for CML include TKIs, which specifically target the ATP-binding site of the Abl domain and inhibit its kinase activity.
Understanding Bcr abl Cancer Protein

The Bcr-Abl cancer protein is a fusion protein that is formed as a result of a genetic abnormality known as the Philadelphia chromosome. This genetic abnormality occurs when parts of the BCR (breakpoint cluster region) gene on chromosome 22 and the ABL (Abelson) gene on chromosome 9 are combined, creating a hybrid gene. This hybrid gene produces a protein known as Bcr-Abl, which is responsible for the development of certain types of leukemia, most notably chronic myelogenous leukemia (CML).
The Bcr-Abl protein functions as a constitutively active tyrosine kinase, meaning that it is always turned on and promotes uncontrolled cell division and growth. This uncontrolled cell division leads to the development of cancer. The Bcr-Abl protein contains several domains that are important for its function, including an N-terminal oligomerization domain, a kinase domain, and a C-terminal regulatory domain.
N-terminal Oligomerization Domain
The N-terminal domain of the Bcr-Abl protein is responsible for its ability to form oligomers, which are complexes composed of multiple Bcr-Abl proteins. Oligomerization is thought to play a role in enhancing the stability and activity of the Bcr-Abl protein, as well as promoting its localization to specific cellular compartments.
Kinase Domain
The kinase domain of the Bcr-Abl protein is responsible for its tyrosine kinase activity. This domain phosphorylates (adds a phosphate group to) tyrosine residues on target proteins, leading to the activation of signaling pathways that promote cell growth and survival. The constitutive activation of the kinase domain in the Bcr-Abl protein is a key feature of its oncogenic function.
C-terminal Regulatory Domain
The C-terminal regulatory domain of the Bcr-Abl protein contains multiple binding sites for various proteins, including negative regulators of tyrosine kinase activity. Mutations in this domain can disrupt the binding of these negative regulators, leading to increased kinase activity and enhanced oncogenic potential of the Bcr-Abl protein.
By understanding the structure and function of the Bcr-Abl protein, scientists have been able to develop targeted therapies that specifically inhibit its activity. These targeted therapies, such as tyrosine kinase inhibitors, have revolutionized the treatment of CML and other Bcr-Abl-positive leukemias, significantly improving patient outcomes.
The Role of Bcr abl in Cancer Development
The Bcr abl protein is a fusion protein that results from a chromosomal translocation between the Bcr gene on chromosome 22 and the abl gene on chromosome 9. This translocation is commonly found in chronic myeloid leukemia (CML) and plays a crucial role in the development and progression of this type of cancer. Understanding the role of Bcr abl in cancer development is important for the development of targeted therapies and treatments.
Bcr abl is an oncogenic protein that possesses both tyrosine kinase activity and signaling capabilities. The fusion of the Bcr and abl genes results in a constitutively active tyrosine kinase, which leads to the deregulation of downstream signaling pathways involved in cell growth, survival, and proliferation. This dysregulated signaling is one of the main drivers of cancer development, as it promotes uncontrolled cell division and inhibits apoptosis, leading to the accumulation of malignant cells.
The Bcr abl protein also plays a role in the development of drug resistance. It has been observed that CML patients who initially respond well to treatment with tyrosine kinase inhibitors, which target the Bcr abl protein, can develop resistance over time. This resistance is often caused by mutations in the Bcr abl gene that result in altered protein structure and function. These mutated forms of Bcr abl are less susceptible to inhibition by the targeted therapies and can continue to promote cancer cell growth and survival.
In conclusion, the Bcr abl protein plays a crucial role in the development and progression of cancer, particularly in chronic myeloid leukemia. It functions as a constitutively active tyrosine kinase, driving dysregulated signaling pathways that promote cell growth and inhibit apoptosis. Understanding the mechanisms underlying the role of Bcr abl in cancer development is essential for the development of targeted therapies that can effectively inhibit its activity and overcome drug resistance.
Investigating the Structure of Bcr abl Protein
The Bcr abl protein is a fusion protein that results from a chromosomal translocation between the BCR (breakpoint cluster region) gene and the ABL (Abelson) gene. This translocation is commonly found in patients with chronic myeloid leukemia (CML) and is responsible for the constitutive activation of the ABL kinase domain, leading to uncontrolled cell proliferation. Understanding the structure of the Bcr abl protein is crucial for developing targeted therapies to treat CML.
The Bcr abl protein consists of several distinct domains, including the Bcr domain, the coiled-coil region, the tyrosine kinase domain, and the SH2 (Src homology 2) and SH3 (Src homology 3) domains. The Bcr domain plays a role in regulating the activity of the ABL kinase domain, while the coiled-coil region mediates dimerization of the Bcr abl protein. The tyrosine kinase domain is responsible for phosphorylating target proteins, while the SH2 and SH3 domains are involved in protein-protein interactions.
Structural studies have revealed that the Bcr abl protein adopts a compact folded conformation, with the tyrosine kinase domain positioned in the center and surrounded by the other domains. The crystal structure of the ABL kinase domain in complex with an ATP analog revealed key residues involved in ATP binding and catalysis. In addition, X-ray crystallography and cryo-electron microscopy have provided insights into the dimerization interface of the Bcr abl protein and its interaction with other proteins, such as the adaptor protein Grb2.
Further investigation of the structure of the Bcr abl protein is essential for understanding its role in CML pathogenesis and for developing more effective targeted therapies. High-resolution structural studies, such as nuclear magnetic resonance spectroscopy and cryo-electron microscopy, can provide detailed information about the dynamic behavior of the protein and its interactions with ligands and other proteins. This knowledge can guide the design of small-molecule inhibitors that specifically target the Bcr abl protein and disrupt its abnormal kinase activity, leading to the development of more personalized and effective treatments for CML.
Key Functional Domains of Bcr abl Protein
The Bcr abl protein is a fusion protein created by the translocation of genetic material between the Bcr gene and the abl gene. This translocation is commonly found in patients with Chronic Myelogenous Leukemia (CML) and is responsible for the constitutive activation of the abl tyrosine kinase. The Bcr abl protein is composed of multiple functional domains, each of which plays a crucial role in the pathogenesis of CML.
One key domain of the Bcr abl protein is the Bcr domain. This domain, derived from the Bcr gene, contains several important subdomains, including the coiled-coil domain, the SH2 domain, and the SH3 domain. The coiled-coil domain is responsible for the oligomerization of the Bcr abl protein, which is necessary for its oncogenic activity. The SH2 domain interacts with phosphotyrosine residues on various cellular proteins, leading to the activation of downstream signaling pathways. The SH3 domain, on the other hand, is involved in protein-protein interactions and helps recruit other signaling molecules to the Bcr abl complex.
Another important domain of the Bcr abl protein is the abl tyrosine kinase domain. This domain, derived from the abl gene, plays a central role in the phosphorylation of target proteins. The constitutive activation of the abl tyrosine kinase in the Bcr abl protein leads to the abnormal phosphorylation of a wide range of cellular substrates, resulting in dysregulated cell growth and survival. Targeted therapies for CML, such as tyrosine kinase inhibitors, specifically target this domain to block its activity and inhibit the growth of leukemia cells.
- The Bcr SH2 domain interacts with phosphotyrosine residues
- The Bcr SH3 domain is involved in protein-protein interactions
- The abl tyrosine kinase domain phosphorylates target proteins
In addition to these domains, the Bcr abl protein also contains other functional motifs, such as the serine/threonine kinase domain, the DNA-binding domain, and the nuclear localization signal. These domains contribute to the diverse functions of the Bcr abl protein, including its role in cell cycle regulation, apoptosis resistance, and genomic instability. Understanding the structure and function of these key domains is crucial for the development of targeted therapies against CML.
Interaction of Bcr abl with Cellular Pathways
The Bcr abl fusion protein is formed as a result of a translocation event between chromosomes 9 and 22, resulting in the fusion of the Bcr gene on chromosome 22 with the abl gene on chromosome 9. This fusion protein has constitutive tyrosine kinase activity, which leads to dysregulation of various cellular signaling pathways.
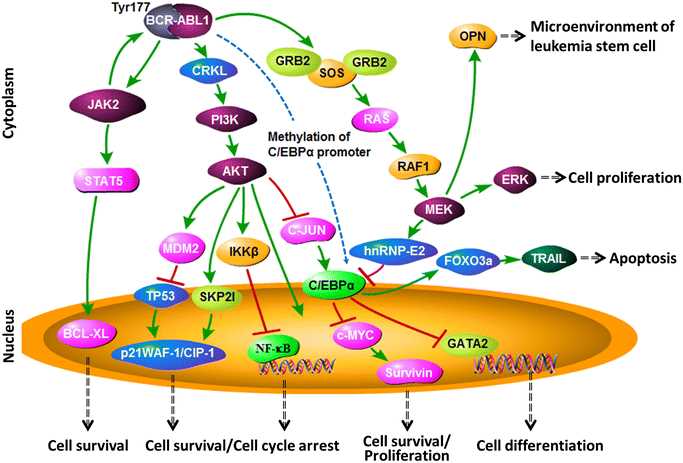
The Bcr abl fusion protein interacts with several cellular pathways, including the Ras/MAPK pathway, the PI3K/Akt pathway, and the JAK/STAT pathway. These interactions play a crucial role in the development and progression of Bcr abl-positive leukemia.
The Ras/MAPK pathway is involved in cell proliferation and survival. The Bcr abl protein activates this pathway by directly phosphorylating and activating Ras, leading to increased cell proliferation and resistance to apoptosis.
The PI3K/Akt pathway is an important regulator of cell growth and survival. The Bcr abl protein activates this pathway by phosphorylating and activating Akt, resulting in increased cell survival and resistance to apoptosis.
The JAK/STAT pathway is involved in the regulation of cell growth and differentiation. The Bcr abl protein activates this pathway by directly phosphorylating and activating JAK2, leading to increased cell proliferation and resistance to differentiation.
Overall, the interaction of the Bcr abl fusion protein with these cellular pathways leads to dysregulation of cell proliferation, survival, and differentiation, contributing to the development and progression of Bcr abl-positive leukemia.