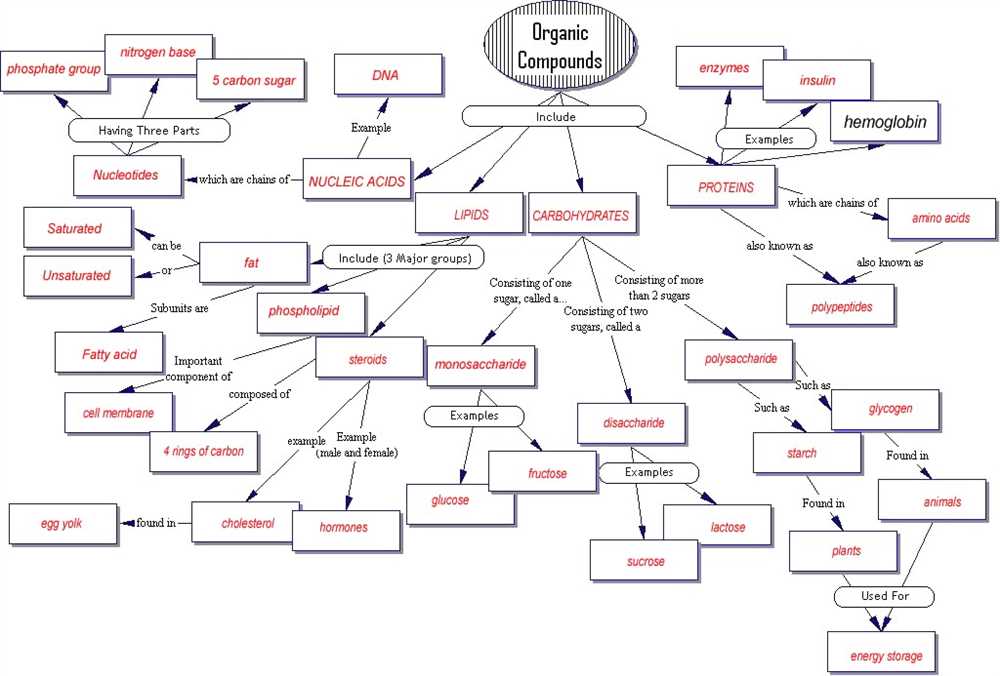
Understanding the key concepts in biochemistry is essential for anyone studying or working in the field of biology. Biochemistry is the branch of science that explores the chemical processes and substances that occur within living organisms. It provides a foundation for understanding how molecules, such as proteins, carbohydrates, and lipids, are synthesized, broken down, and interact with each other.
A concept map is a visual representation of the connections and relationships between various biochemical processes and molecules. It helps to organize and understand complex information by illustrating how different concepts and principles are interconnected. In this article, we will provide an answer key to a biochemistry concept map, highlighting the main ideas and connections between different biochemistry topics.
Some of the key topics covered in the biochemistry concept map answer key include: the structure and function of biomolecules such as proteins, nucleic acids, carbohydrates, and lipids; the central dogma of molecular biology, which describes the flow of genetic information from DNA to RNA to protein; enzyme kinetics and catalysis, including the factors that influence enzyme activity; and metabolic pathways, such as glycolysis, the citric acid cycle, and oxidative phosphorylation.
By studying and understanding the biochemistry concept map answer key, students and professionals in the field of biology can gain a deeper understanding of the intricate biochemical processes that occur within living organisms. This knowledge is crucial for many applications in areas such as medicine, agriculture, biotechnology, and environmental science.
Biochemistry Concept Map Answer Key: Unlocking the Secrets of Molecular Interactions
The study of biochemistry explores the intricate world of molecular interactions within living organisms. Understanding how molecules interact with one another is essential for deciphering the fundamental processes that govern life, from metabolism to cell signaling. Biochemists have developed various analytical tools and techniques to unravel the complex web of molecular interactions, and the biochemistry concept map serves as a key to unlocking the secrets of these interactions.
Analyzing Molecular Interactions:
The biochemistry concept map answer key provides a comprehensive overview of the different types of molecular interactions, such as protein-protein interactions, protein-DNA interactions, and enzyme-substrate interactions. It highlights the key players in these interactions, including amino acids, nucleic acids, and small molecules, and illustrates how their specific properties and structures contribute to the stability and specificity of the interaction.
The concept map also delves into the various forces that govern molecular interactions, such as hydrogen bonding, electrostatic interactions, and hydrophobic interactions. It emphasizes the importance of these forces in determining the strength and specificity of the interactions and how slight alterations in the molecular structure can have profound effects on the outcome of the interaction.
Applications in Drug Discovery and Medicine:
The biochemistry concept map answer key is not only a tool for understanding the fundamental principles of molecular interactions but also a valuable resource for drug discovery and medicine. It provides insights into the molecular mechanisms underlying diseases and offers potential targets for therapeutic intervention.
By analyzing the concept map, researchers can identify key molecules or pathways that are dysregulated in diseases and design targeted drugs to modulate these interactions. For example, understanding the protein-protein interactions involved in cancer cell signaling pathways can lead to the development of small molecule inhibitors that disrupt these interactions and halt tumor growth.
Ultimately, the biochemistry concept map answer key serves as a guide for scientists and researchers, enabling them to navigate the complex world of molecular interactions and uncover the mysteries of life at the molecular level.
The Basics of Biochemistry
Biochemistry is the study of the chemical processes and substances that occur within living organisms. It is a combination of biology and chemistry, focusing on the molecular aspects of life. Understanding biochemistry is crucial in many fields, including medicine, agriculture, and environmental sciences, as it provides a foundation for understanding how biological systems function.
Molecules: At the core of biochemistry are molecules, the building blocks of life. These molecules include proteins, nucleic acids, carbohydrates, and lipids. Each of these molecules has a specific structure and function within living organisms. For example, proteins are involved in nearly every aspect of cellular function, from cell signaling to enzyme catalysis. Nucleic acids, such as DNA and RNA, carry genetic information and are essential for the synthesis of proteins. Carbohydrates are a source of energy for the body, while lipids play a role in energy storage and cell membrane composition.
- Enzymes: Enzymes are proteins that catalyze biochemical reactions in the body. They act as biological catalysts, increasing the rate of chemical reactions by lowering the activation energy required for the reaction to occur. Enzymes are highly specific and only catalyze specific reactions. They play a crucial role in metabolism, the process by which living organisms obtain and utilize energy from their environment.
- Metabolism: Metabolism refers to the chemical reactions that occur in the body to maintain life. It involves the breakdown of molecules to release energy and the synthesis of molecules for growth and repair. Metabolism is divided into two main types: catabolism, the breakdown of molecules, and anabolism, the synthesis of molecules. The regulation of metabolism is tightly controlled to ensure that the body’s energy needs are met.
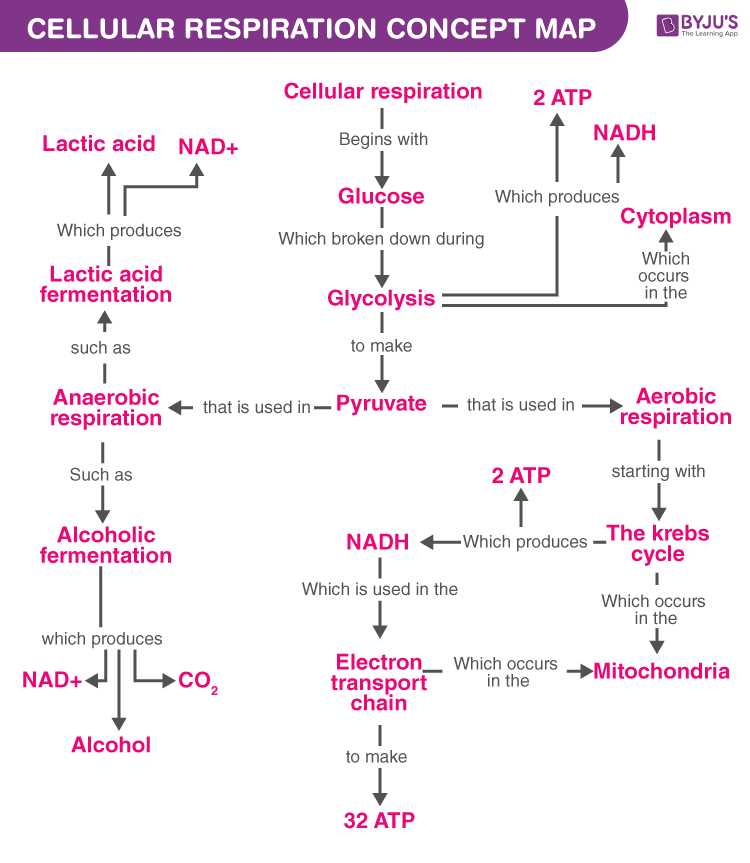
- Cellular Respiration: Cellular respiration is the process by which cells convert glucose and oxygen into carbon dioxide, water, and energy. The energy generated by cellular respiration is stored as adenosine triphosphate (ATP), the energy currency of the cell. ATP is used to power various cellular processes, such as muscle contraction, nerve impulse transmission, and active transport across cell membranes.
In conclusion, biochemistry is a complex and multidisciplinary field that studies the chemical processes within living organisms. It involves the study of molecules, enzymes, metabolism, and cellular respiration. Understanding the basics of biochemistry is essential for advancing our knowledge in various scientific disciplines and improving our understanding of life itself.
Understanding Molecular Interactions
Molecular interactions play a crucial role in various biological processes and are essential for the functioning of living organisms. These interactions involve the binding of molecules through different types of forces, such as hydrogen bonding, van der Waals forces, electrostatic interactions, and hydrophobic interactions. By understanding the underlying principles of molecular interactions, scientists can gain insights into the structure, function, and dynamics of biological molecules.
Hydrogen bonding: Hydrogen bonding is a type of molecular interaction in which a hydrogen atom of one molecule forms a weak bond with an electronegative atom of another molecule. This type of interaction is important in maintaining the structure of biomolecules such as proteins and nucleic acids. It also plays a crucial role in the recognition and binding of ligands to receptors in processes like signal transduction.
Van der Waals forces: Van der Waals forces are weak, short-range forces that arise due to the fluctuating electron distribution around atoms and molecules. These forces include dipole-dipole interactions, dipole-induced dipole interactions, and London dispersion forces. Van der Waals forces are important in stabilizing the three-dimensional structure of proteins and in the binding of small molecules to proteins.
Electrostatic interactions: Electrostatic interactions occur between charged molecules or between charged and uncharged molecules. These interactions can be attractive or repulsive depending on the charges involved. They play a crucial role in a variety of biological processes, such as enzyme-substrate interactions, protein-protein interactions, and ion-channel interactions.
Hydrophobic interactions: Hydrophobic interactions are the tendency of nonpolar molecules or groups to cluster together in the presence of water. This phenomenon is driven by the energetically favorable organization of water molecules around hydrophobic molecules. Hydrophobic interactions play a crucial role in protein folding, protein-protein interactions, and the formation of lipid bilayers.
In conclusion, understanding molecular interactions is fundamental in unraveling the complexities of biochemistry. By studying the forces and interactions that govern biological molecules, scientists can gain insights into the structure, function, and interactions of living systems.
Key Elements in Biochemical Reactions
In biochemistry, an essential aspect of understanding cellular processes is the study of biochemical reactions. These reactions involve the transformation of molecules to achieve specific biological functions within cells. While there are various elements involved in these reactions, certain key components play a crucial role in catalyzing and regulating the process.
Enzymes
- Enzymes are biological catalysts that speed up chemical reactions without being consumed themselves.
- They are proteins that have specific active sites where substrates bind, leading to the formation of product(s).
- Enzymes play a vital role in regulating biochemical reactions by controlling the rate at which they occur.
Substrates
- Substrates are the molecules that react with enzymes to form product(s).
- They bind to the active site of the enzyme through complementary shapes and chemical interactions.
- Each enzyme has a specific substrate that it recognizes and acts upon.
Products
- Products are the molecules formed as a result of biochemical reactions.
- They can be the end products of a metabolic pathway or serve as starting materials for subsequent reactions.
- The formation of products is essential for the functioning and survival of cells.
Cofactors and coenzymes
- Cofactors and coenzymes are non-protein molecules that assist enzymes in catalyzing biochemical reactions.
- Cofactors can be inorganic ions, such as magnesium or zinc, while coenzymes are organic molecules, such as vitamins.
- These molecules bind to enzymes to facilitate the transfer of chemical groups or electrons during the reaction.
Energy
- Energy is required for biochemical reactions to occur and for the formation of products.
- Activation energy is the energy needed to start a reaction, and enzymes lower this energy barrier, allowing reactions to proceed efficiently.
- Energy is also released or absorbed during biochemical reactions, depending on whether they are exergonic (release energy) or endergonic (require energy).
Understanding the key elements involved in biochemical reactions is crucial for comprehending cellular processes, developing new therapies, and exploring the mechanisms underlying various diseases.
Enzymes: Nature’s Catalysts
Enzymes play a crucial role in the field of biochemistry as nature’s catalysts. These specialized proteins facilitate and accelerate biochemical reactions by lowering the activation energy required for a reaction to occur. By doing so, enzymes enable living organisms to carry out essential metabolic processes at a significantly faster rate than would be possible without their presence.
One key characteristic of enzymes is their high specificity. Each enzyme is tailored to catalyze a particular reaction by recognizing and binding to specific substrates. This specificity is due to the unique three-dimensional structure of the enzyme’s active site, which complements the shape and chemical composition of the substrate. Once the enzyme and substrate bind together, the enzyme can effectively perform its catalytic function, transforming the substrate into product and releasing it.
Enzymes achieve catalysis through various mechanisms, including facilitating molecular rearrangements, breaking down or building up chemical bonds, and transferring functional groups between molecules. These mechanisms often involve a series of intermediate steps referred to as the enzyme-substrate complex. The enzymatic reaction is highly efficient, allowing enzymes to catalyze reactions at rates millions of times faster than their uncatalyzed counterparts.
Enzymes can be regulated in several ways to ensure optimal functioning within an organism. The activity of enzymes can be modulated through feedback inhibition, where the end product of a metabolic pathway binds to an enzyme earlier in the pathway, inhibiting its activity. Additionally, enzymes can be regulated through allosteric modulation, where small molecules bind to the enzyme, either activating or inhibiting its function. These regulatory mechanisms allow organisms to coordinate and adjust metabolic processes in response to changing environmental conditions.
- Enzymes serve as nature’s catalysts, accelerating biochemical reactions by lowering activation energy.
- Enzymes are highly specific, recognizing and binding to specific substrates through their unique active sites.
- Enzymes achieve catalysis through various mechanisms, facilitating molecular rearrangements, bond breakdown, and functional group transfer.
- Enzymes can be regulated through feedback inhibition and allosteric modulation to ensure optimal functioning.
Metabolism: The Key to Energy Balance
The process of metabolism is essential for maintaining energy balance in living organisms. Metabolism refers to the chemical reactions that occur within cells to convert food into energy. These reactions can be divided into two main categories: catabolism and anabolism.
Catabolism is the breakdown of complex molecules into simpler ones, releasing energy in the process. This energy is used to fuel the activities of cells and tissues. Key processes involved in catabolism include glycolysis, the Krebs cycle, and oxidative phosphorylation. Glycolysis breaks down glucose into pyruvate, generating ATP molecules. The Krebs cycle then further breaks down pyruvate, producing additional energy-rich molecules. Finally, oxidative phosphorylation occurs in the mitochondria, where electrons from energy-rich molecules are used to generate ATP.
On the other hand, anabolism is the synthesis of complex molecules from simpler ones, requiring energy. This process allows cells to build and repair structures, such as proteins, nucleic acids, and cell membranes. Anabolic reactions are essential for growth, tissue repair, and the production of hormones and enzymes. Examples of anabolic processes include the synthesis of proteins from amino acids and the synthesis of DNA and RNA from nucleotides.
To maintain energy balance, the rates of catabolism and anabolism must be balanced. When the body requires additional energy, such as during physical activity, catabolic processes are activated to break down stored molecules and release energy. Conversely, when the body has excess energy, anabolic processes are stimulated to store energy in the form of glycogen or fat.
In conclusion, metabolism plays a crucial role in energy balance by converting food into energy and building or repairing cellular structures. The balance between catabolism and anabolism ensures that energy is available when needed and stored when there is an excess. Understanding the intricacies of metabolism is essential for maintaining overall health and well-being.